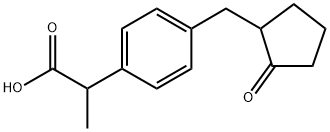
Loxoprofen
Synonym(s):α-Methyl-4-[(2-oxocyclopentyl)methyl]benzeneacetic acid;Koloxo
- CAS NO.:68767-14-6
- Empirical Formula: C15H18O3
- Molecular Weight: 246.31
- MDL number: MFCD00864331
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is Loxoprofen?
Absorption
Loxoprofen is rapidly and completely absorbed from the GI tract with a bioavailability of 95%. The absorption phase of the medication occurs in the first 4-6 hours after ingestion. Food ingestion with the medication causes a slight decrease in the rate of loxoprofen absorption.
Toxicity
Adverse effects include anorexia, nausea, vomiting, bleeding, anemia, diarrhea, and constipation. Loxoprofen toxicity may lead to gastrointestinal disturbance (including flatulence, dyspepsia and gastritis) and renal failure.
The Uses of Loxoprofen
Loxoprofen is a non-selective nonsteroidal anti-inflammatory drug (NSAID) that has been effective in reducing atherosclerosis in mice by reducing inflammation. Loxoprofen becomes active after metabolism in the body and inhibits the activation of cyclooxygenase.
The Uses of Loxoprofen
antiinflammatory, analgesic
Indications
Loxoprofen is non-steroidal anti-inflammatory medication (NSAID) indicated for pain and inflammation related to musculoskeletal and joint disorders. In addition to its effects on pain, it is an antipyretic and anti-inflammatory medication.
Background
Loxoprofen is a propionic acid derivative non-steroidal anti-inflammatory drug. It is marketed under the trade name Loxonin in Brazil, Mexico and Japan by Sankyo, as Loxomac in India, and as Oxeno in Argentina. A transdermal preparation was approved for use in Japan in January 2006.
What are the applications of Application
Loxoprofen is a non-steroidal anti-inflammatory compound
Definition
ChEBI: A monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-[(2-oxocyclopentyl)methyl]phenyl group. A prodrug that is rapidly converted to its active trans-alcohol metabolite following ora administration.
Pharmacokinetics
Loxoprofen is a non-selective inhibitor of cyclooxygenase enzymes, which are responsible for the formation of various biologically active pain, fever, and inflammatory mediators. These include prostaglandins, prostacyclin, thromboxane, and arachidonic acid.
Metabolism
Loxoprofen is a prodrug that is rapidly converted to its active trans-alcohol metabolite by carbonyl reductase in the liver. This same process also results in a cis-alcohol metabolite, though this isomer carries little pharmacological activity. The parent drug has also been observed to undergo oxidation via CYP3A4/5 to two hydroxylated metabolites (M3 and M4) and glucuronidation by UGT2B7 to two glucuronide metabolites (M5 and M6). The alcohol metabolites of loxoprofen also undergo glucuronide conjugation via UGT2B7 to two glucuronide metabolites (M7 and M8) prior to excretion.
When applied in topical formulations, loxoprofen is metabolized to its active trans-alcohol form by carbonyl reductase in the skin.
Properties of Loxoprofen
| Melting point: | 108.5-111° |
| Boiling point: | bp0.3 190-195° |
| Density | 1.182±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | solid |
| pka | 4.39±0.10(Predicted) |
| color | White to Off-White |
| Merck | 14,5589 |
| InChI | InChI=1S/C15H18O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13H,2-4,9H2,1H3,(H,17,18) |
| CAS DataBase Reference | 68767-14-6(CAS DataBase Reference) |
Safety information for Loxoprofen
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Loxoprofen
| InChIKey | YMBXTVYHTMGZDW-UHFFFAOYSA-N |
| SMILES | C(C1CCCC1=O)C1C=CC(C(C)C(=O)O)=CC=1 |
Loxoprofen manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





![2-[(4-Acetylphenyl)methyl]cyclopentan-1-one](https://img.chemicalbook.in/CAS/20180703/GIF/96824-28-1.gif)


You may like
-
 Loxoprofen 98%View Details
Loxoprofen 98%View Details -
 68767-14-6 99%View Details
68767-14-6 99%View Details
68767-14-6 -
 Loxoprofen 98%View Details
Loxoprofen 98%View Details
68767-14-6 -
 Loxoprofen 95% CAS 68767-14-6View Details
Loxoprofen 95% CAS 68767-14-6View Details
68767-14-6 -
 Loxoprofen CAS 68767-14-6View Details
Loxoprofen CAS 68767-14-6View Details
68767-14-6 -
 Loxoprofen CAS 68767-14-6View Details
Loxoprofen CAS 68767-14-6View Details
68767-14-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
