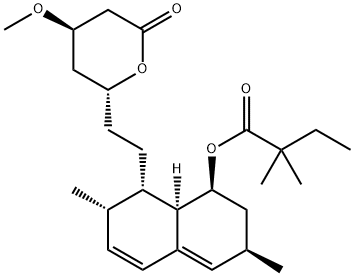
Lovastatin
Synonym(s):2-Methyl-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester butanoic acid;6-α-Methylcompactin;Lovastatin;Mevinolin;Monacolin K
- CAS NO.:75330-75-5
- Empirical Formula: C24H36O5
- Molecular Weight: 404.54
- MDL number: MFCD00072164
- EINECS: 616-212-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
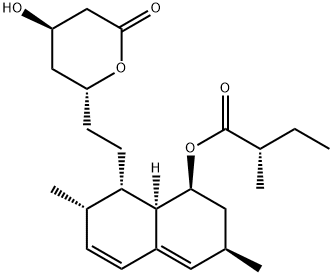
What is Lovastatin?
Absorption
Lovastatin Cmax was found to be 3.013ng/mL with a Tmax of 3.36 hours.
Plasma concentrations of total radioactivity (lovastatin plus 14C-metabolites) peaked at 2 hours and declined rapidly to about 10% of peak by 24 hours postdose. Absorption of lovastatin, estimated relative to an intravenous reference dose, in each of four animal species tested, averaged about 30% of an oral dose. In animal studies, after oral dosing, lovastatin had high selectivity for the liver, where it achieved substantially higher concentrations than in non-target tissues. Lovastatin undergoes extensive first-pass extraction in the liver, its primary site of action, with subsequent excretion of drug equivalents in the bile. As a consequence of extensive hepatic extraction of lovastatin, the availability of drug to the general circulation is low and variable. In a single dose study in four hypercholesterolemic patients, it was estimated that less than 5% of an oral dose of lovastatin reaches the general circulation as active inhibitors. Following administration of lovastatin tablets the coefficient of variation, based on between-subject variability, was approximately 40% for the area under the curve (AUC) of total inhibitory activity in the general circulation.
The peak concentrations of lovastatin when a dose of 10-40 mg is administered are reported to range from 1.04-4.03 ng/ml and an AUC of 14-53 ng.h/ml. This indicates that lovastatin presents a dose-dependent pharmacokinetic profile. When lovastatin was given under fasting conditions, plasma concentrations of both active and total inhibitors were on average about two-thirds those found when lovastatin was administered immediately after a standard test meal.
Genetic differences in the OATP1B1 (Organic-Anion-Transporting Polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact lovastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) showed that lovastatin Cmax and AUC were 340 and 286% higher, respectively, for individuals homozygous for 521CC compared to homozygous 521TT individuals. The 521CC genotype is also associated with a marked increase in the risk of developing myopathy, likely secondary to increased systemic exposure. Other statin drugs impacted by this polymorphism include rosuvastatin, pitavastatin, atorvastatin, simvastatin, and pravastatin.
While specific dosage instructions are not included in the available product monographs for lovastatin, individuals with the above-mentioned c.521CC OATP1B1 genotype should be monitored for development of adverse effects from increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis, particularly at higher doses.
Toxicity
The median lethal dose of lovastatin is higher than 15 g/m2. Five healthy human volunteers have received up to 200 mg of lovastatin as a single dose without clinically significant adverse experiences. A few cases of accidental overdosage have been reported; no patients had any specific symptoms, and all patients recovered without sequelae. The maximum dose taken was 5 to 6 g.
In carcinogenic studies, there is an increase in the incidence of hepatocellular carcinomas and adenomas, pulmonary adenomas, papilloma in non-glandular mucose in stomach and thyroid neoplasms. However, with respect to effects on fertility, lovastatin has been reported to present testicular atrophy, decreased spermatogenesis, spermatocytic degeneration and giant cell formation which derived into decreased fertility in males. Lastly, there is no evidence of mutagenicity induced by lovastatin.
Description
In 1987, lovastatin became the first statin drug (HMG-CoA reductase inhibitor) to be approved by the US Food and Drug Administration for lowering blood cholesterol levels. It was first isolated from the fungus?Aspergillus terreus?in the 1970s; it is now manufactured by a fermentation process. It is no longer patent-protected, but it is still widely known by its brand name, Mevacor.
Description
Lovastatin is an orally-active hypocholesterolemic useful in the treatment of familial hypercholesterolemia. The drug acts by inhibiting the HMG-CoA reductase-catalyzed rate limiting step of cholesterol biosynthesis. While treatment with lovastatin leads to significant reductions in total and LDL cholesterol, its effect on atherosclerosis or coronary heart disease has not been established.
Chemical properties
White Solid
Physical properties
Appearance: White, nonhygroscopic crystalline powder. Solubility: Insoluble in water (0.0004?mg/mL); sparingly soluble in ethanol, methanol, isobutanol, isopropanol, acetonitrile, n-propanol; soluble in acetone, N-dimethylformamide; freely soluble in chloroform. Melting point: 174.5?°C. Specific optical rotation: 25?°C for D (sodium) line, +323° (concentration 0.5? g in 100? ml acetonitrile). Stability: Lovastatin is sensitive to light. Following exposure to extreme light conditions, the drug is stable for 24?h or 1?month when exposed to UV (approximately 3230 lux) or fluorescent (approximately 10,764 lux) light, respectively, at 28?°C in air.
Originator
Merck (USA)
History
Statins are the most extensively used class of lipid-lowering medication. Lovastatin
is the second statin discovered by scientists.
Later, the official name lovastatin was established. The activity of lovastatin is
much better than compactin.
In July 1982, lovastatin showed dramatic effects on lowering LDL cholesterol in
patients with severe hypercholesterolemia who were unresponsive to the existedmedicines, with very few adverse reactions .
The Uses of Lovastatin
Lovastatin (mevinolin) is a metabolite first isolated from Monascus ruber and later found in several other fungal species. Lovastatin is a potent inhibitor of HMG-CoA. HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, responsible for the biosynthesis of cholesterol. Lovastatin was developed as a drug as a hypolipemic agent.
The Uses of Lovastatin
An antihypercholesterolemic agent. A fungal metabolite, which is a potent inhibitor of HMG-CoA reductase
The Uses of Lovastatin
antiarrhythmic
The Uses of Lovastatin
anti-hyperlipoproteinemic, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor
The Uses of Lovastatin
Cardiovascular
Background
Lovastatin, also known as the brand name product Mevacor, is a lipid-lowering drug and fungal metabolite derived synthetically from a fermentation product of Aspergillus terreus. Originally named Mevinolin, lovastatin belongs to the statin class of medications, which are used to lower the risk of cardiovascular disease and manage abnormal lipid levels by inhibiting the endogenous production of cholesterol in the liver. More specifically, statin medications competitively inhibit the enzyme hydroxymethylglutaryl-coenzyme A (HMG-CoA) Reductase, which catalyzes the conversion of HMG-CoA to mevalonic acid and is the third step in a sequence of metabolic reactions involved in the production of several compounds involved in lipid metabolism and transport including cholesterol, low-density lipoprotein (LDL) (sometimes referred to as "bad cholesterol"), and very low-density lipoprotein (VLDL). Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD, such as those with Type 2 Diabetes. The clear evidence of the benefit of statin use coupled with very minimal side effects or long term effects has resulted in this class becoming one of the most widely prescribed medications in North America.
Lovastatin and other drugs from the statin class of medications including atorvastatin, pravastatin, rosuvastatin, fluvastatin, and simvastatin are considered first-line options for the treatment of dyslipidemia. Increasing use of the statin class of drugs is largely due to the fact that cardiovascular disease (CVD), which includes heart attack, atherosclerosis, angina, peripheral artery disease, and stroke, has become a leading cause of death in high-income countries and a major cause of morbidity around the world. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
While all statin medications are considered equally effective from a clinical standpoint, rosuvastatin is considered the most potent; doses of 10 to 40mg rosuvastatin per day were found in clinical studies to result in a 45.8% to 54.6% decrease in LDL cholesterol levels, while lovastatin has been found to have an average decrease in LDL-C of 25-40%. Potency is thought to correlate to tissue permeability as the more lipophilic statins such as lovastatin are thought to enter endothelial cells by passive diffusion, as opposed to hydrophilic statins such as pravastatin and rosuvastatin which are taken up into hepatocytes through OATP1B1 (organic anion transporter protein 1B1)-mediated transport. Despite these differences in potency, several trials have demonstrated only minimal differences in terms of clinical outcomes between statins.
What are the applications of Application
Lovastatin is a hypocholesterolemic HMGCR inhibitor (statin that lowers cholesterol levels)
Indications
Lovastatin is indicated to reduce the risk of myocardial infarction, unstable angina, and the need for coronary revascularization procedures in individuals without symptomatic cardiovascular disease, average to moderately elevated total-C and LDL-C, and below average HDL-C. It is indicated as an intervention alternative in individuals presenting dyslipidemia at risk of developing atherosclerotic vascular disease. The administration of this agent should be accompanied by the implementation of a fat and cholesterol-restricted diet.
Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Lovastatin is indicated as an adjunct to diet for the reduction of elevated total-C and LDL-C levels in patients with primary hypercholesterolemia (Types IIa and IIb2), when the response to diet restricted in saturated fat and cholesterol and to other nonpharmacological measures alone has been inadequate.
Lovastatin is also indicated to slow the progression of coronary atherosclerosis in patients with coronary heart disease as part of a treatment strategy to lower total-C and LDL-C to target levels.
Lovastatin is indicated as an adjunct to diet to reduce total-C, LDL-C and apolipoprotein B levels in adolescent boys and girls with Heterozygous Familial Hypercholesterolemia (HeFH) who are at least one year post-menarche, 10 to 17 years of age, with HeFH if after an adequate trial of diet therapy the following findings are present: LDL-C remains greater than 189 mg/dL or LDL-C remains greater than 160 mg/dL and there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the adolescent patient.
Before administering lovastatin, it is important to rule out the presence of secondary causes of hypercholesterolemia and a lipid profile should be performed.
Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Indications
Hypercholesterolemia, combined hyperlipidemia.
Definition
ChEBI: Lovastatin is a fatty acid ester that is mevastatin carrying an additional methyl group on the carbobicyclic skeleton. It is used in as an anticholesteremic drug and has been found in fungal species such as Aspergillus terreus and Pleurotus ostreatus (oyster mushroom). It has a role as an Aspergillus metabolite, a prodrug, an anticholesteremic drug and an antineoplastic agent. It is a polyketide, a statin (naturally occurring), a member of hexahydronaphthalenes, a delta-lactone and a fatty acid ester. It is functionally related to a (S)-2-methylbutyric acid and a mevastatin.
Manufacturing Process
1) Coniothyrium fuckelii ATCC 74227 was grown in a sterilizable fermentation apparatus with a volume of 15 L. The apparatus was equipped with an agitator, aerator, pH control system, dissolved oxygen control system, and a pump and feed system designed to allow the sterile addition of glucose solutions. The pH was controlled by the automatic addition of ammonium hydroxide or phosphoric acid to maintain the pH of the culture medium constant at 5.0. Periodically, the fermentation broth was sampled, measured for glucose concentration and an addition of glucose was made manually to maintain a concentration of glucose at approximately 2-5 g/L. After 192 hours of growth under these conditions, the concentration of biomass reached 65 g/L and the concentration of Lovastatin reached 102 mg/L.
2) A further medium for the growth of Coniothyrium fuckelii ATCC 74227, has the following composition: Glucose 12%, Peptone 1%, (NH4)2SO4 0.4%, MgSO4 · 7H2O 0.05%, P 2000 0.1% (Antifoam agent), L-isoleucine 0.2-1.5%, L-aspartic acid 0.2-1.5%. The fermentation was carried out as before.
With this medium, the lovastatin concentration was 430 mg/L.
brand name
Altoprev (Andrx); Mevacor (Merck).
Therapeutic Function
Antihyperlipidemic
General Description
Lovastatin, 2-methylbutanoic acid 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester, mevinolin,MK-803 (Mevacor) (formerly called mevinolin), is a potentinhibitor of HMG-CoA. The drug was obtained originallyfrom the fermentation products of the fungi Aspergillus terreusand Monascus ruber. Lovastatin was one of two originalHMG-CoA reductase inhibitors. The other drug, mevastatin(formerly called compactin), was isolated from cultures ofPenicillium cillium citrum. Mevastatin was withdrawn fromclinical trials because it altered intestinal morphology in dogs.This effect was not observed with lovastatin. For inhibitoryeffects on HMG-CoA reductase, the lactone ring must be hydrolyzedto the open-ring heptanoic acid.
Biological Activity
Potent, competitive inhibitor of HMG-CoA reductase (K i = 0.6 nM) therefore decreases cholesterol biosynthesis, in vitro and in vivo . Decreases CDK2, 4, 6 and cyclin E levels and induces G1 arrest and apoptosis in tumor cell lines in vitro .
Pharmacokinetics
Lovastatin is an oral antilipemic agent which reversibly inhibits HMG-CoA reductase. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, lovastatin reduces the risk of cardiovascular morbidity and mortality.
Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks. Clinical studies have shown that lovastatin reduces LDL-C and total cholesterol by 25-40%. The 50% inhibitory dose is known to be of 46 mcg/kg which is translated into a reduction of approximately 30% of plasma cholesterol.
Myopathy/Rhabdomyolysis
Lovastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is dose-related and is increased by high levels of HMG-CoA reductase inhibitory activity in plasma. In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20 to 40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily.
Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may also be at increased risk for myopathy. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued.
The risk of myopathy during treatment with lovastatin may be increased with concurrent administration of interacting drugs such as fenofibrate, niacin, gemfibrozil, cyclosporine, and strong inhibitors of the CYP3A4 enzyme. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors coadministered with colchicine, and caution should therefore be exercised when prescribing these two medications together.
Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment.
Liver Dysfunction
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials. When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. In the EXCEL study, the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages.
Pharmacology
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
(HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to
mevalonate , a rate-determining step of cholesterol biosynthesis. Lovastatin is
metabolized as a prodrug into an active form, lovastatin acid. Lovastatin acid is a
reversible competitive inhibitor for HMG-CoA.
However, the reduction in plasma cholesterol by statins is not only due to reduction in cholesterol biosynthesis. .
In addition to their lipid-lowering properties, statins produce several nonlipidrelated properties, include decreasing levels of high-sensitivity C-reactive protein
(hsCRP), improving endothelial function, reducing inflammation at the site of the
atherosclerotic plaque, inhibiting platelet aggregation, anticoagulant, etc. .
Clinical Use
The primary uses of lovastatin are the treatment of dyslipidemia and the prevention of cardiovascular disease. It is recommended to be used only when other approaches such as diet, exercise, and weight reduction have not improved the cholesterol profile (“Lovastatin”. The American Society of Health-System Pharmacists. Retrieved 3 April 2011.). Lovastatin is useful in treating hypercholesterolemia and combined hyperlipidemia. However, lovastatin is not effective in treatment of receptornegative homozygous familial hypercholesterolemia .
Side Effects
Lovastatin's common side effects are listed in approximately descending order of occurrence frequency: creatine phosphokinase elevation, flatulence, abdominal pain, constipation, diarrhea, muscle aches or pains, nausea, indigestion, weakness, blurred vision, rash, dizziness, and muscle cramps. As with all statin drugs, it can rarely cause myopathy, hepatotoxicity (liver damage), dermatomyositis, or rhabdomyolysis.
Metabolism
Lovastatin is given as a lactone prodrug and thus, in order to produce its mechanism of action, it is required to be converted to the active beta-hydroxy form. This drug activation process does not seem to be related to CYP isoenzyme activity but rather to be controlled by the activity of serum paraoxonase.
Lovastatin is metabolized by the microsomal hepatic enzyme system (Cytochrome P-450 isoform 3A4). The major active metabolites present in human plasma are the β-hydroxy acid of lovastatin, its 6'-hydroxy, 6'-hydroxymethyl, and 6'-exomethylene derivatives. The uptake of lovastatin by the liver is enhanced by the activity of OATP1B1.
Storage
Store at RT
References
1) Alberts et al. (1988), Discovery, biochemistry and biology of lovastatin;? Am. J. Cardiol., 62 10J 2) Hancock et al. (1989), All ras proteins are polyisoprenylated but only some are palmitoylated;? Cell, 57 1167 3) Park et al. (1999), Lovastatin-induced inhibition of HL-60 cell proliferation via cell cycle arrest and apoptosis;? Anticancer Res., 19 3133 4) Vilimanovich et al. (2015), Statin-mediated inhibition of cholesterol synthesis induces cytoprotective autophagy in human leukemic cells;? Eur. J. Pharmacol., 765 415 5) Tobert et al. (1988), Efficacy and long-term adverse effect pattern of lovastatin;? Am. J. Cardiol., 62 28J
Properties of Lovastatin
| Melting point: | 175°C |
| Boiling point: | 559.2±50.0 °C(Predicted) |
| alpha | D25 +323° (c = 0.5 g in 100 ml acetonitrile) |
| Density | 1.12±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 320 ° (C=0.5, CH3CN) |
| storage temp. | -20°C |
| solubility | ethanol: soluble9.80-10.20mg/mL, clear, colorless to faintly yellow |
| form | White to off-white powder |
| pka | 13.49±0.40(Predicted) |
| color | White |
| Water Solubility | 0.0004 mg/mL at 25 ºC |
| Merck | 14,5586 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 75330-75-5(CAS DataBase Reference) |
| EPA Substance Registry System | Lovastatin (75330-75-5) |
Safety information for Lovastatin
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Lovastatin
Lovastatin manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Lovastatin 98%View Details
Lovastatin 98%View Details
75330-75-5 -
 LOVASTATIN CALCIUM 95-99%View Details
LOVASTATIN CALCIUM 95-99%View Details
75330-75-5 -
 Lovastatin, 98% CAS 75330-75-5View Details
Lovastatin, 98% CAS 75330-75-5View Details
75330-75-5 -
 Lovastatin CAS 75330-75-5View Details
Lovastatin CAS 75330-75-5View Details
75330-75-5 -
 Lovastatin 95.00% CAS 75330-75-5View Details
Lovastatin 95.00% CAS 75330-75-5View Details
75330-75-5 -
 Lovastatin 98% (HPLC) CAS 75330-75-5View Details
Lovastatin 98% (HPLC) CAS 75330-75-5View Details
75330-75-5 -
 Lovastatin >98% (HPLC) CAS 75330-75-5View Details
Lovastatin >98% (HPLC) CAS 75330-75-5View Details
75330-75-5 -
 Lovastatin 98% (HPLC) CAS 75330-75-5View Details
Lovastatin 98% (HPLC) CAS 75330-75-5View Details
75330-75-5
