Lithium acetylide ethylenediamine complex
- CAS NO.:6867-30-7
- Empirical Formula: C4H7LiN2
- Molecular Weight: 90.05
- MDL number: MFCD00013183
- EINECS: 229-967-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
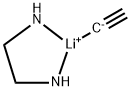
What is Lithium acetylide ethylenediamine complex?
Chemical properties
Lithium Acetylide Ethylenediamine Complex is a slightly yellow to light brown crystals, Lithium acetylide is an unstable solid which disproportionates to form lithium carbide (dilithium acetylide) and acetylene. It is soluble in liquid ammonia at its boiling point to the extent of just over one mole per liter. It is not sensitive to shock and presents a hazard due only to possible caustic burns and the hazards presented by ammonia and acetylene, which form when the material contacts the air.
The Uses of Lithium acetylide ethylenediamine complex
Lithium acetylide ethylenediamine complex is used in the synthesis of a chiral cyclopropanol. It is also used as an alkynylating reagent for the enantioselective alkynylation of ketones catalyzed by lithium binaphtholate without using other metal sources.
The Uses of Lithium acetylide ethylenediamine complex
The largest industrial use of lithium acetylide is in the production of vitamin A. It is used to ethynylate methyl vinyl ketone to produce a tertiary carbinol, an intermediate in the multistep synthesis.
A second industrial use is in the production of Placidyl®, a tranquilizer . In this case it is used to ethynylate methyl beta-chloro vinyl ketone. Lithium acetylide is used extensively in the laboratory, mostly for the ethynylation of ketones.
Lithium acetylide is not an article of commerce since it is unstable and cannot be shipped.
The Uses of Lithium acetylide ethylenediamine complex
- Lithium acetylide, ethylenediamine complex is a general reagent to synthesize ethynylated ketones.
- It is used as a ligand in the coordination chemistry to synthesize transition metal complexes such as 1-alkynyl-dimethyl(triorganophosphine)gold(III) complex.
- It is employed in the ring opening reaction of epoxides, as in the total synthesis of (?)-goniotrionin and englerin A.
- It can also be used in the ethynylation of alkyl halides to prepare terminal alkynes.
Production Methods
Lithium acetylide is produced both in the laboratory and industrially by dissolving lithium metal in refluxing liquid ammonia and then bubbling acetylene into the solution. The solution of monolithium acetylide thus formed is stabilized by the complexing of the lithium ion with ammonia forming a polar acetylenic anion which does not react further with lithium metal. However, if the reaction is carried out in tetrahydrofuran, for example, the lithium acetylide reacts further with lithium to form dilithium acetylide (lithium carbide). In fact, if the ammonia is removed from a solution of lithium acetylide, it disproportionates to dilithium acetylide and acetylene.
Preparation
The ethylenediamine complex of lithium acetylide is produced by allowing lithium metal to react with ethylenediamine in benzene at reflux to form AMithioethylenediamine: H2NCH2CH2NH2+Li -> L1HNCH2CH2NH2+05H2.
Properties of Lithium acetylide ethylenediamine complex
| Melting point: | 76 °C (dec.) |
| Boiling point: | 110.6 °C (lit.) |
| Density | 1,04 g/cm3 |
| Flash point: | 7 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in ethylene diamine, n-propyl amine, n-butyl amine, pyridine, dimethyl formamaide, diglyme, dioxan and dimethylsulfoxide. |
| form | Crystals or Crystalline Powder |
| color | Slightly yellow to light brownor |
| Sensitive | Moisture Sensitive |
| BRN | 3593824 |
| CAS DataBase Reference | 6867-30-7(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium, (1,2-ethanediamine-.kappa.N,.kappa.N')ethynyl- (6867-30-7) |
Safety information for Lithium acetylide ethylenediamine complex
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Lithium acetylide ethylenediamine complex
| InChIKey | QJQWXTYPTBEPGS-UHFFFAOYSA-N |
Lithium acetylide ethylenediamine complex manufacturer
JSK Chemicals
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Lithium acetylide, ethylenediamine complex, 90% 99%View Details
Lithium acetylide, ethylenediamine complex, 90% 99%View Details
6867-30-7 -
 Lithium acetylide ethylenediamine complex CAS 6867-30-7View Details
Lithium acetylide ethylenediamine complex CAS 6867-30-7View Details
6867-30-7 -
 Lithium acetylide CAS 6867-30-7View Details
Lithium acetylide CAS 6867-30-7View Details
6867-30-7 -
 Lithium acetylide, ethylenediamine complex CAS 6867-30-7View Details
Lithium acetylide, ethylenediamine complex CAS 6867-30-7View Details
6867-30-7 -
 Lithium Acetylide PowderView Details
Lithium Acetylide PowderView Details
6867-30-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
