lisdexamfetamine
- CAS NO.:608137-32-2
- Empirical Formula: C15H25N3O
- Molecular Weight: 263.38
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-26 17:16:27
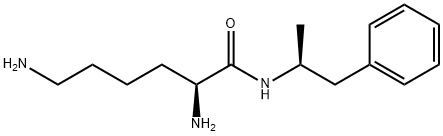
What is lisdexamfetamine?
Absorption
After oral administration, lisdexamfetamine dimesylate is rapidly absorbed from the gastrointestinal tract. Following single-dose oral administration of lisdexamfetamine in pediatric patients with ADHD under fasted conditions, Tmax of lisdexamfetamine and dextroamphetamine was reached at approximately one hour and 3.5 hours post-dose, respectively. Weight/Dose normalized AUC and Cmax values were the same in pediatric patients as the adults. Food prolongs Tmax by approximately one hour and may decrease the exposure (Cmax and AUC) of dextroamphetamine.
Toxicity
The LD50 value for lisdexamfetamine dihydrochloride in rats was >1000 mg/kg.
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has been reported with amphetamine use, including lisdexamfetamine. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Convulsions and coma usually precede fatal poisoning. Lisdexamfetamine and d-amphetamine are not dialyzable.
Description
Lisdexamfetamine—trade name Vyvanse—is amphetamine coupled with l-lysine. It is being developed to treat attention-deficit hyperactivity disorder (ADHD) in pediatric patients. It provides controlled release of amphetamine via hydrolysis within the body and decreases the potential for abuse because crushing the capsule does not accelerate its activation.
The Uses of lisdexamfetamine
Treatment of atten tion deficit hyperactivity disorder (ADHD).
Indications
Lisdexamfetamine is indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in adults and pediatric patients six years and older. It is also indicated to treat moderate to severe binge eating disorder (BED) in adults. It is approved for use in the US and Canada.
Background
Lisdexamfetamine is a prodrug of dextroamphetamine, a central nervous system stimulant known as d-amphetamine, covalently attached to the naturally occurring amino acid L-lysine. Lisdexamfetamine is the first chemically formulated prodrug stimulant and was first approved by the FDA in April 2008. It was also approved by Health Canada in February 2009. Lisdexamfetamine works to treat attention deficit hyperactivity disorder and binge eating disorder by blocking dopamine and norepinephrine reuptake and increasing their levels in the extraneuronal space.
Definition
ChEBI: Lisdexamfetamine is an amino acid amide.
Pharmacokinetics
Once administered, lisdexamfetamine is converted to its active metabolite, dextroamphetamine, which is taken up by the brain. Dextroamphetamine blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increases the release of these monoamines into the extraneuronal space. The parent drug, lisdexamfetamine, does not bind to the sites responsible for the reuptake of norepinephrine and dopamine in vitro.
Metabolism
Lisdexamfetamine dimesylate is hydrolyzed by red blood cells to dextroamphetamine and l-lysine primarily in the blood. Substantial hydrolysis occurs even at low hematocrit levels. Dextroamphetamine can further be metabolized to form other metabolites, such as hippuric acid.
Lisdexamfetamine is not metabolized by cytochrome P450 enzymes.
Properties of lisdexamfetamine
| Boiling point: | 488.0±45.0 °C(Predicted) |
| Density | 1.045±0.06 g/cm3(Predicted) |
| pka | 15.11±0.46(Predicted) |
Safety information for lisdexamfetamine
Computed Descriptors for lisdexamfetamine
lisdexamfetamine manufacturer
Zenfold Sustainable Technologies (ZST)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 Lisdexamfetamine 99%View Details
Lisdexamfetamine 99%View Details -
 608137-32-2 98%View Details
608137-32-2 98%View Details
608137-32-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1