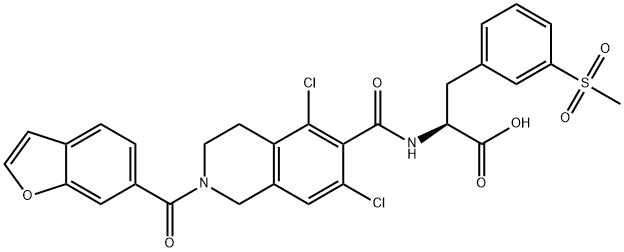
lifitegrast
- CAS NO.:1025967-78-5
- Empirical Formula: C29H24Cl2N2O7S
- Molecular Weight: 615.48
- MDL number: MFCD28502439
- EINECS: 813-044-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
What is lifitegrast?
Absorption
The mean peak plasma concentration (Cmax) of 1.70ng/mL was reached within 15 minutes of application. Quantifiable trough plasma concentrations ranged from 0.55 ng/mL to 3.74 ng/mL . Observations show limited systemic exposure that produces significant clinical outcomes.
Toxicity
Some adverse effects in 5-25% of the patients include instillation site irritation, dysgeusia and reduced visual acuity. There is no evidence or reports of potential carcinogenic, mutagenic or fertility-altering effects of this drug.
Description
Lifitegrast (Xiidra; lifitegrast sodium; SAR1118; SHP606; SPD606) is a lymphocyte functionassociated antigen-1 (LFA-1) antagonist. It inhibits T-cell inflammation by blocking the binding of two key surface proteins (LFA-1 and ICAM-1) that mediate the chronic inflammatory cascade associated with dry eye disease. In a phase III clinical trial, lifitegrast 5% ophthalmic solution (50 mg/mL) is administered as a single 0.2mL eye drop twice a day into each eye for an 84 day treatment period.

Lifitegrast does not currently have Marketing Authorisation in the EU for any indication. Lifitegrast is licensed for use in the USA for treatment of the signs and symptoms of dry eye disease. The most common adverse reactions (incidence 5-25%) reported following the use of lifitegrast are instillation site irritation, dysgeusia, and decreased visual acuity.
Chemical properties
Lifitegrast is a white to off-white powder which is soluble in water.
The Uses of lifitegrast
Lifitegrast is used for the treatment of signs and symptons of dry eye diseases. It also Inhibites corneal inflammation that is capable of causing pains, blurred vision and ocular discomfort in sufferer.
Background
Lifitegrast is a FDA approved drug for the treatment of keratoconjunctivitis sicca (dry eye syndrome). It is a tetrahydroisoquinoline derivative and lymphocyte function-associated antigen-1 ( LFA-1) antagonist that was discovered through the rational design process. The ophthalmic solution was approved in July, 2016 under the trade name Xiidra. It has shown to protect the corneal surface and alleviate the symptoms of dry eye syndrome with fast onset of action and well tolerated profile in both local and systemic setting .
Indications
For the treatment of signs and symptoms of keratoconjunctivitis sicca (dry eye syndrome).
Definition
ChEBI: An N-acyl-L-alpha-amino acid obtained by formal condensation of the carboxy group of N-[2-(1-benzofuran-6-carbonyl)]-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid with he amino group of 3-(methanesulfonyl)-L-phenylalanine. Used for treatment of keratoconjunctivitis sicca (dry eye syndrome).
Indications
Topical lifitegrast was approved by the FDA for the treatment of dry eye. Lifitegrast decreases inflammation by blocking the interaction between intercellular adhesion molcule 1 and lymphocyte function- -associated antigen 1. In four, large, multicellular, randomized clinical trials, lifitegrast was shown to be effective in improving the signs and symptoms of dry eye. The side effects of lifitegrast include transient ocular irritation and dysgeusia. Further studies are needed to explore the effectiveness of combination therapy such as the concomitant use of topical cyclosporine and topical liftegrast.
Mechanism of action
Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in dry eye disease. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell activation and migration to target tissues. In vitro studies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines in human peripheral blood mononuclear cells. The exact mechanism of action of lifitegrast in dry eye disease is not known.
Pharmacokinetics
Lifitegrast addresses both the symptoms and the resulting ocular surface damage by interfering with ocular inflammatory cycle . Lifitegrast is a lymphocyte function–associated antigen-1 antagonist through direct competitive antagonism and sequentially inhibits the T-cell recruitment, activation, and proinflammatory cytokine release associated with dry eye syndrome .
Pharmacokinetics
In a subset of dry eye disease patients (n=47) enrolled in a Phase 3 trial, the pre-dose (trough) plasma concentrations of lifitegrast were measured after 180 and 360 days of topical ocular dosing (1 drop twice daily) with Xiidra (lifitegrast ophthalmic solution) 5%. A total of nine (9) of the 47 patients (19%) had plasma lifitegrast trough concentrations above 0.5 ng/mL (the lower limit of assay quantitation). Trough plasma concentrations that could be quantitated ranged from 0.55 ng/mL to 3.74 ng/mL.
Side Effects
A multicenter, randomized, double-masked, placebo-controlled phase 3 study (n = 331) evaluating the safety of lifitegrast ophthalmic solution for the treatment of dry eye disease reported the most common non-ocular effect was dysgeusia (change in taste) occurring in 16.4% of patients in the lifitegrast group and 1.8% of the placebo group.
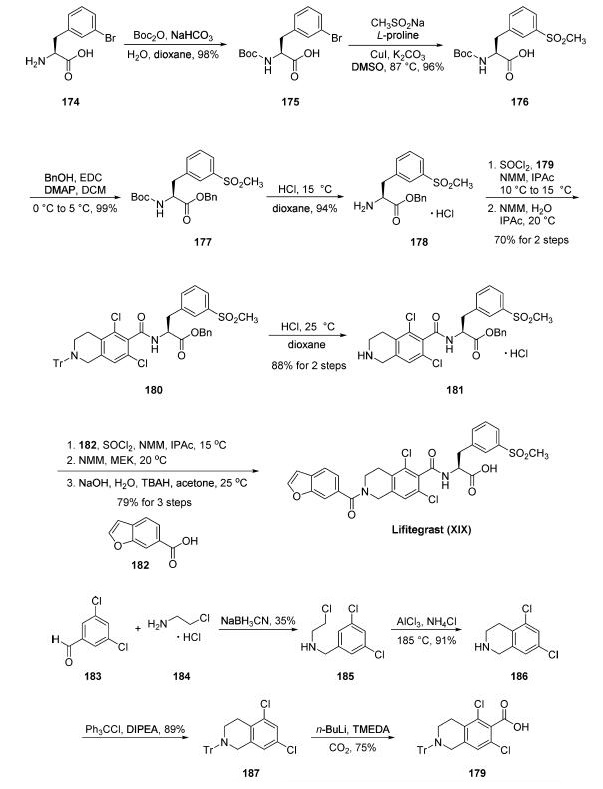
Synthesis
After Boc protection, reaction with sodium
methanesulfinate in the presence of copper iodide, K2CO3,
and L-proline gave rise to sulfonate 176. Esterification of 176
with benzyl alcohol followed by removal of the Boc group
within 177 yielded the corresponding HCl salt of the
aminoester 178. Amide bond coupling with acid 179 furnished amide 180, which was then
subjected to 4 N HCl in dioxane resulting in trityl removal and
arrival at HCl salt 181 in 88% yield over two steps.
Tetrahydroisoquinoline 181 was then coupled with commercial
benzofuranyl acid 182 to give rise to lifitegrast benzyl ester in
90% yield. Finally, saponification delivered lifitegrast (XIX) in
88% yield.
Tetrahydroisoquinoline-6-carboxylic acid 179 was prepared
starting from commercial 3,5-dichlorobenzaldehyde 183. Reductive amination with 1-chloro-2-aminoethane
184 gave chloroethyl amine 185, which underwent an
efficient intramolecular Friedel-Crafts reaction using AlCl3 to
generate the corresponding tetrahydroisoquinoline 186 in 91%
yield. N-Tritylation of 186 proceeded in 89% yield, and this was followed by a directed o-metalation reaction and carbon dioxide
quench to furnish the requisite acid 179 in 75% yield.
Metabolism
Based on the findings of an in vitro metabolism study using fresh human hepatocytes, lifitegrast does not appear to undergo significant metabolism .
Properties of lifitegrast
| Melting point: | >163°C (dec.) |
| Boiling point: | 811.9±65.0 °C(Predicted) |
| Density | 1.479±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Sonicated) |
| form | Solid |
| pka | 3.14±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for lifitegrast
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for lifitegrast
lifitegrast manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![L-Phenylalanine, N-[(5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester, hydrochloride (1:1)](https://img.chemicalbook.in/CAS/20211123/GIF/1194550-65-6.gif)

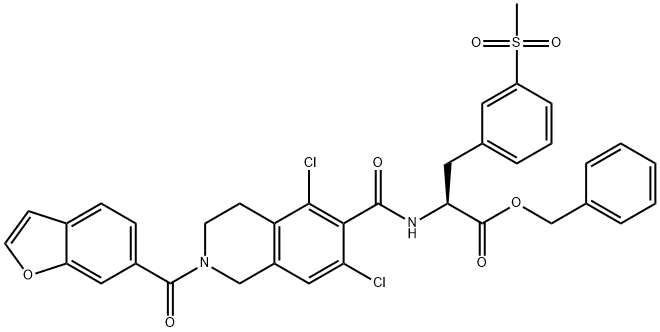
![2(1H)-Isoquinolinecarboxylic acid, 5,7-dichloro-3,4-dihydro-6-[[[(1S)-1-[[3-(methylsulfonyl)phenyl]methyl]-2-oxo-2-(phenylmethoxy)ethyl]amino]carbonyl]-, 1,1-dimethylethyl ester](https://img.chemicalbook.in/CAS/20180703/GIF/1194550-61-2.gif)
You may like
-
 1025967-78-5 Lifitegrast 98%View Details
1025967-78-5 Lifitegrast 98%View Details
1025967-78-5 -
 1025967-78-5 98%View Details
1025967-78-5 98%View Details
1025967-78-5 -
 Lifitegrast 98%View Details
Lifitegrast 98%View Details
1025967-78-5 -
 Lifitegrast 1025967-78-5 99%View Details
Lifitegrast 1025967-78-5 99%View Details
1025967-78-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
