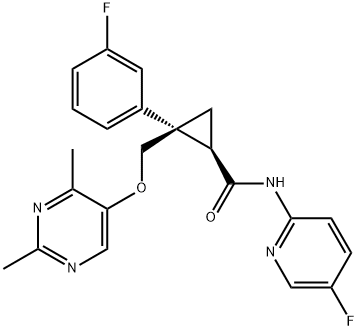
Lemborexant
- CAS NO.:1369764-02-2
- Empirical Formula: C22H20F2N4O2
- Molecular Weight: 410.42
- MDL number: MFCD28502271
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-24 13:33:21
What is Lemborexant?
Absorption
Animal models of lemborexant disposition have demonstrated rapid absorption following oral administration. The Tmax of lemborexant is approximately 1-3 hours, or 3-5 hours following administration of a high-fat, high-calorie meal. Cmax and AUC0-24h increase at a rate slightly less than proportionate to the given dose. Following administration of a high-fat, high-calorie meal, Cmax is decreased by 23% and AUC0-inf is increased by 18%. AUC, Cmax, and terminal half-life are increased in the presence of moderate hepatic impairment, and AUC (but not half-life) is increased in the presence of mild hepatic impairment.
Toxicity
Clinical experience with lemborexant overdose is limited. In clinical studies, healthy patients receiving doses up to 10x the recommended maximum dose experienced dose-dependent increases in the frequency of adverse effects such as somnolence - it is likely, then, that symptoms of overdose will be consistent with lemborexant's adverse effect profile. In the event of an overdosage, implement supportive measures and consult the nearest poison control center for the most up to date management strategies. As lemborexant is highly protein-bound, hemodialysis is likely to be of little use in overdose situations.
Description
Lemborexant is a newer orexin receptor antagonist which is submitted to US FDA for review as new drug application for the treatment of insomnia after completing two key Phase 3 studies of lemborexant – SUNRISE 1 (Study 304) and SUNRISE 2 (Study 303).
Chemical properties
Lemborexant is a white to off-white powder that is practically insoluble in water.
The Uses of Lemborexant
Lemborexant is a novel orexin receptor antagonist and is used in the treatment of insomnia, characterised by difficulties with sleep onset or sleep maintenance. Orexins are neuropeptides involved in regulating sleep and arousal by promoting wakefulness. Lemborexant blocks the binding of orexins A and B to their receptors 1 and 2 thereby reducing wakefulness and promoting sleep. Suvorexant is the other orexin receptor antagonist marketed in Australia for insomnia.
Background
Lemborexant is a novel dual orexin receptor antagonist used in the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. Recent research in the field of sleep disorders has revealed that insomnia is likely driven not by the inability of the brain to "switch on" sleep-related circuits, but rather an inability to "switch-off" wake-promoting circuits. Whereas historically popular pharmacologic treatments for insomnia (e.g. zopiclone, zolpidem, benzodiazepines) focus on enhancing sleep drive via modulation of GABA and melatonin receptors, lemborexant and other orexin antagonists (e.g. suvorexant) act to counteract inappropriate wakefulness. This novel mechanism of action offers potential advantages over classic hypnotic agents, including a more favorable adverse effect profile and potentially greater efficacy, and may signal the beginning of a new wave of treatment options for patients suffering from insomnia.
Indications
Lemborexant is indicated for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance.
Pharmacokinetics
Lemborexant promotes sleep by antagonizing the actions of wake-promoting chemicals in the brain. Episodes of complex sleep behaviors (e.g. eating food, having sex, making phone calls) have been reported in patients using lemborexant - these events may occur in hypnotic-naive and hyponotic-experienced patients, and patients are unlikely to remember these events. Patients exhibiting complex sleep behaviors should discontinue lemborexant immediately. Lemborexant may carry some risk of abuse, and should be used with caution in patients with a history of alcohol or drug addiction. Its controlled substance schedule is currently under review by the Drug Enforcement Administration.
Pharmacokinetics
| Effect of food | High-fat & high-calorie meal delay Tmax & reduce Cmax |
| Tmax | 1 to 3 hours |
| Half-life | 17 hours (5 mg) and 19 hours (10 mg) |
| Metabolism | primarily metabolized by CYP3A4 |
| Excretion | Urine (29%); feces (57%) |
Drug interactions
Lemborexant is metabolized by CYP3A4 and is susceptible to interactions with medications such as amiodarone, amlodipine, carbamazepine, clarithromycin, diltiazem, fluoxetine and phenytoin. Lemborexant may affect other medications metabolised by CYP2B6, such as bupropion and methadone. Alcohol should be avoided: a single dose of alcohol can increase lemborexant concentrations up to 70%.
Metabolism
Given that less than 1% of an administered dose is recovered unchanged in the urine, it is likely that lemborexant is extensively metabolized - this has been confirmed in rat and monkey models, but its metabolism in humans has not been fully characterized. Prescribing information states that it is predominantly metabolized by CYP3A4, with a smaller contribution by CYP3A5. The major circulating metabolite is lemborexant's M10 metabolite, which is pharmacologically active and binds to orexin receptors with a similar affinity to the parent drug. The M10 metabolite has the potential to induce CYP3A and CYP2B6 enzymes, weakly inhibit CYP3A enzymes, and is a substrate of P-gp transporters.
Mode of action
The mechanism of action of lemborexant in the treatment of insomnia is Blocks the binding of wake-promoting neuropeptides orexin A and orexin B to receptors OX1R and OX2R, presumed to suppress wake drive.
References
https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212028s000lbl.pdf
https://www.smchealth.org/sites/main/files/file-attachments/dayvigo_for_publish.pdf?1592332002
https://www.superiorhealthplan.com/content/dam/centene/Superior/policies/pharmacy-policies/Lemborexant%20(Dayvigo)%20(CP.PMN.233)%20(PDF).pdf
Properties of Lemborexant
| Melting point: | 173 - 175°C |
| Boiling point: | 596.1±50.0 °C(Predicted) |
| Density | 1.347±0.06 g/cm3(Predicted) |
| storage temp. | -20°C, Inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.30±0.40(Predicted) |
| color | White to Off-White |
Safety information for Lemborexant
Computed Descriptors for Lemborexant
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1369764-02-2 Lemborexant API 98%View Details
1369764-02-2 Lemborexant API 98%View Details
1369764-02-2 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4