LEAD STEARATE
- CAS NO.:7428-48-0
- Empirical Formula: C36H70O4Pb
- Molecular Weight: 774.1386
- EINECS: 231-068-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 13:50:14
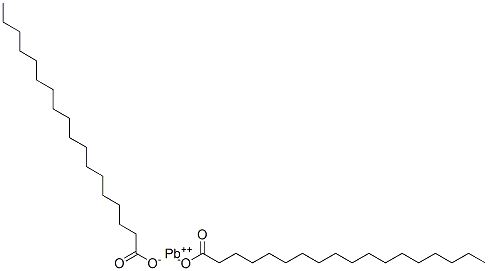
What is LEAD STEARATE?
Description
Lead stearate is an organic lead compound. Itis a white powder with a slight fatty odor. Molecularweight = 1734.87; Freezing/Melting point = 116℃; Flashpoint = 232℃. Hazard Identification (based on NFPA-704M Rating System): Health 2, Flammability 1, Reactivity 0.Insoluble in water.
Chemical properties
Lead stearate is an organic lead compound. It is a white powder with a slight fatty odor.
Potential Exposure
It is used as a stabilizer for plastics and rubber processing; in extreme-pressure lubricants and as a drier in varnishes
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Administer saline cathartic and anenema. For relief of colic, administer antispasmodic (calcium gluconate, atropine, papaverine). Consider morphinesulfate for severe pain.Whole blood lead levels, circulating plasma/erythrocytelead concentration ratio, urine ALA, and erythrocyte protoporphyrin fluorescent microscopy may all be useful in monitoring or assessing lead exposure. Chelating agents, such asedetate disodium calcium (Ca EDTA) and penicillamine(not penicillin), are generally useful in the therapy of acutelead intoxication.Antidotes and special procedures for lead: Persons with significant lead poisoning are sometimes treated with CaEDTA while hospitalized. This “chelating” drug causes arush of lead from the body organs into the blood and kidneys, and thus has its own hazards, and must be administered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA orsimilar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from heat, oxidizers, strong acids.Dust may explode at high temperature. Lead is regulated byan OSHA Standard 1910.1025. All requirements of the standard must be followed.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Incompatibilities
Dust may form explosive mixture with air; keep away from high heat and sources of ignition. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides
Properties of LEAD STEARATE
| Melting point: | 125°C |
| Density | 1.4000 |
| EPA Substance Registry System | Lead stearate (7428-48-0) |
Safety information for LEAD STEARATE
Computed Descriptors for LEAD STEARATE
Abamectin manufacturer
Pallav Chemicals And Solvents Pvt Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidYou may like
-
 Lead Stearate Extrapure 7428-48-0 99%View Details
Lead Stearate Extrapure 7428-48-0 99%View Details
7428-48-0 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4