LACTOSE, MONOHYDRATE
- CAS NO.:10039-26-6
- Empirical Formula: C12H24O12
- Molecular Weight: 360.31
- MDL number: MFCD00166994
- EINECS: 600-087-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-25 18:47:31
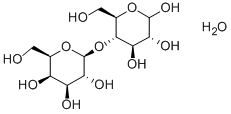
What is LACTOSE, MONOHYDRATE?
Chemical properties
White or almost white, crystalline powder.
Chemical properties
In the solid state, lactose appears as various isomeric forms,
depending on the crystallization and drying conditions, i.e. alactose
monohydrate, b-lactose anhydrous, and a-lactose anhydrous.
The stable crystalline forms of lactose are a-lactose
monohydrate, b-lactose anhydrous, and stable a-lactose anhydrous.
Lactose occurs as white to off-white crystalline particles or
powder. Lactose is odorless and slightly sweet-tasting; a-lactose is
approximately 20% as sweet as sucrose, while b-lactose is 40% as
sweet.
The Uses of LACTOSE, MONOHYDRATE
Excipient.
Production Methods
Lactose is a natural disaccharide consisting of galactose and
glucose, and is present in the milk of most mammals. Commercially,
lactose is produced from the whey of cows’ milk; whey being the residual liquid of the milk following cheese and casein production.
Cows’ milk contains 4.4–5.2% lactose; lactose constitutes 38% of
the total solid content of milk.
a-Lactose monohydrate is prepared by crystallization from
supersaturated solutions below 93.5°C. Various crystalline shapes
are prism, pyramidal, and tomahawk; these are dependent on the
method of precipitation and crystallization. Direct compression
grades of a-lactose monohydrate are prepared by granulation/
agglomeration and spray-drying.
Pharmaceutical Applications
Lactose is widely used as a filler and diluent in tablets and capsules,
and to a more limited extent in lyophilized products and infant
formulas. Lactose is also used as a diluent in dry-powder
inhalation; seeLactose, Inhalation. Various lactose grades are
commercially available that have different physical properties
such as particle size distribution and flow characteristics. This
permits the selection of the most suitable material for a particular
application; for example, the particle size range selected for capsules
is often dependent on the type of encapsulating machine used.
Usually, fine grades of lactose are used in the preparation of tablets
by the wet-granulation method or when milling during processing is
carried out, since the fine size allows better mixing with other
formulation ingredients and utilizes the binder more efficiently.
Other applications of lactose include use in lyophilized products,
where lactose is added to freeze-dried solutions to increase plug size
and aid cohesion. Lactose is also used in combination with sucrose
(approximately 1 : 3) to prepare sugar-coating solutions. It may also
be used in intravenous injections.
Lactose is also used in the manufacture of dry powder
formulations for use as aqueous film-coating solutions or suspensions.
Direct-compression grades of lactose monohydrate are available
as granulated/agglomerated a-lactose monohydrate, containing
small amounts of anhydrous lactose.
Direct-compression grades are often used to carry lower
quantities of drug and this permits tablets to be made without
granulation.
Other directly compressible lactoses are spray-dried lactose and
anhydrous lactose; see Lactose, Spray-Dried and Lactose, Anhydrous.
Safety
Lactose is widely used in pharmaceutical formulations as a filler and
filler-binder in oral capsule and tablet formulations. It may also be
used in intravenous injections. Adverse reactions to lactose are
largely attributed to lactose intolerance, which occurs in individuals
with a deficiency of the intestinal enzyme lactase. This results
in lactose being undigested and may lead to cramps, diarrhea,
distension, and flatulence. In lactose-tolerant individuals, lactase
hydrolyzes lactose in the small intestine to glucose and galactose,
which are then absorbed. Lactase levels are normally high at birth,
and levels decline rapidly in early childhood. Malabsorption of
lactose (hypolactasia) may occur at an early age (4–8 years) and
varies among different ethnic groups. Lactose is excreted unchanged
when administered intravenously.
The symptoms of lactose intolerance are caused by the osmotic
effect of the unabsorbed lactose, which increases water and sodium
levels in the lumen. Unabsorbed lactose, upon reaching the colon,
can be fermented by colonic flora, which produces gas, causing
abdominal distension and discomfort. A lactose tolerance test has
been developed based on the measurement of blood glucose level
and the hydrogen level in the breath. However, its usefulness has
been questioned as the test is based on a 50 g dose of lactose.
Approximately 10–20% of lactose-intolerant individuals, in two
studies, showed clinical symptoms of intolerance after ingestion of
3–5 g of lactose. In one of the studies, 75% of the subjects
had symptoms with 12 g of lactose (equivalent to 250 mL of milk).
In another, eight out of 13 individuals developed diarrhea after
the administration of 20 g of lactose, and nine out of 13 after the
administration of 25 g.
Lower doses of lactose produce fewer adverse effects, and lactose
is better tolerated if taken with other foods. As a result, there is a
significant population with lactose malabsorption who are still able
to ingest normal amounts of lactose, such as that in milk, without
the development of adverse side effects.
Most adults consume about 25 g of lactose per day (500mL of
milk) without symptoms. When symptoms appear, they are
usually mild and dose-related. The dose of lactose in most
pharmaceuticals seldom exceeds 2 g per day. It is unlikely that
severe gastrointestinal symptoms can be attributed to the lactose in
a conventional oral solid-dosage form, especially in adults who have
not previously been diagnosed as severely lactose-intolerant.
However, anecdotal reports of drug-induced diarrhea due to lactose
intolerance have been made following administration of pharmaceutical
preparations containing lactose.
It has also been suggested that lactose intolerance may have a
role in irritable bowel syndrome, but this role is currently
unclear.
In the past, there have been concerns over the transmissible
spongiform encephalopathies (TSE) contamination of animalderived
products. However, in the light of current scientific
knowledge, and irrespective of geographical origin, milk and milk
derivatives are reported as unlikely to present any risk of TSE
contamination; TSE risk is negligible if the calf rennet is produced in
accordance with regulations.
LD50 (rat, IP): >10 g/kg
LD50 (rat, oral): >10 g/kg
LD50 (rat, SC): >5 g/kg
Storage
Mold growth may occur under humid conditions (80% relative
humidity and above). Lactose may develop a brown coloration on
storage, the reaction being accelerated by warm, damp conditions. The purities of different lactoses can vary and color
evaluation may be important, particularly if white tablets are being
formulated. The color stabilities of various lactoses also differ.
Solutions show mutarotation.
Lactose should be stored in a well-closed container in a cool, dry
place.
Incompatibilities
A Maillard-type condensation reaction is likely to occur between
lactose and compounds with a primary amine group to form brown,
or yellow-brown-colored products. The Maillard interaction has
also been shown to occur between lactose and secondary amine.
However, the reaction sequence stops with the formation of the
imine, and no yellow-brown coloration develops.
Lactose is also incompatible with amino acids, amfetamines,
and lisinopril.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (IM, IV, and SC: powder for injections; oral: capsules and tablets; inhalation preparations; vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of LACTOSE, MONOHYDRATE
| Melting point: | ~215 °C (dec.) |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Solid |
| CAS DataBase Reference | 10039-26-6(CAS DataBase Reference) |
Safety information for LACTOSE, MONOHYDRATE
Computed Descriptors for LACTOSE, MONOHYDRATE
LACTOSE, MONOHYDRATE manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![BETA-GAL-[1->3]-BETA-GLCNAC-[1->3]-BETA-GAL-[1-4][ALPHA-FUC-(1->3)]-BETA-GLCNAC-[1->3]-BETA-GAL-[1->4]-GLC](https://img.chemicalbook.in/CAS/GIF/115236-58-3.gif)

You may like
-
 Lactose, monohydrate, GR 99%+ CAS 10039-26-6View Details
Lactose, monohydrate, GR 99%+ CAS 10039-26-6View Details
10039-26-6 -
 Lactose (SQ) CAS 10039-26-6View Details
Lactose (SQ) CAS 10039-26-6View Details
10039-26-6 -
 Lactose, monohydrate, 99% CAS 10039-26-6View Details
Lactose, monohydrate, 99% CAS 10039-26-6View Details
10039-26-6 -
 Lactose Monohydrate sterile (gamma irradiated) CASView Details
Lactose Monohydrate sterile (gamma irradiated) CASView Details -
 Lactose Standard 5 % CAS 10039-26-6View Details
Lactose Standard 5 % CAS 10039-26-6View Details
10039-26-6 -
 D-Lactose monohydrate CAS 10039-26-6View Details
D-Lactose monohydrate CAS 10039-26-6View Details
10039-26-6 -
 LACTOSE MONOHYDRATE Extra Pure CAS 10039-26-6View Details
LACTOSE MONOHYDRATE Extra Pure CAS 10039-26-6View Details
10039-26-6 -
 LACTOSE MONOHYDRATE AR CAS 10039-26-6View Details
LACTOSE MONOHYDRATE AR CAS 10039-26-6View Details
10039-26-6
