L-Menthol
Synonym(s):(-)-Menthol;(1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol;2-Isopropyl-5-methylcyclohexanol;(?)-Menthol;(1R,2S,5R)-(?)-Menthol
- CAS NO.:2216-51-5
- Empirical Formula: C10H20O
- Molecular Weight: 156.27
- MDL number: MFCD00062979
- EINECS: 218-690-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
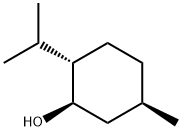
What is L-Menthol?
Toxicity
Menthol, DL: ORAL (LD50): Acute: 2900 mg/kg [Rat], 3100 mg/kg [Mouse]. DERMAL (LD50): Acute: 5001 mg/kg Rabbit.
Chemical properties
white to light yellow crystal powde
The Uses of L-Menthol
(1R,2S,5R)-(-)-Menthol (L-Menthol) is the natural form of Menthol. L-Menthol is used as: refreshing agent, food flavor, cool and antipruritic drug, carminative drug. Menthol crystals is used for pers onal care and cosmetics.
The Uses of L-Menthol
L-Menthol is used as a cooling agent that strongly activates TRPM8.(Transient Receptor Potential Cation Channel, Subfamily M, Member 8 is a Protein Coding gene). It is used as analgesic (topical), antipruritic agent. It is used as: refreshing agent, food flavor, cool and antipruritic drug, carminative drug. Menthol crystals is used for pers onal care and cosmetics.
Indications
Used to treat occasional minor irritation, pain, sore mouth, and sore throat as well as cough associated with a cold or inhaled irritants.
Background
Menthol is a covalent organic compound made synthetically or obtained from peppermint or other mint oils. Forming clear or white waxy, crystalline substance, menthol is typically solid at room temperature. (-)-Menthol is the naturally-occurring and main form of menthol, and is assigned the (1R,2S,5R) configuration. Menthol mediates anesthetic properties and anti-irritating properties locally, thus it is widely used to relieve minor throat irritations.
What are the applications of Application
(?)-Menthol is a cooling agent that strongly activates TRPM8
Definition
ChEBI: A p-menthan-3-ol which has (1R,2S,5R)-stereochemistry. It is the most common naturally occurring enantiomer.
Acquired resistance
(1R,2S,5R)-(-)-Menthol (L-Menthol) is the natural form of Menthol. L- and D-menthol are variants of the same molecule, but have a mirror-image structure in relation to each other, like the right and left hand.
General Description
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate.
Flammability and Explosibility
Non flammable
Biological Activity
l-Menthol inhibite the binding of 13 ligands (calcium channels, sodium channels, γ-aminobutyric acid type A (GABAA) receptor, GABA transporter, dopamine transporter, dopamine D4 receptor, adenosine A2a receptor, α2A-adrenergic receptor, histamine H2 receptor, bombesin receptor, angiotensin AT1 receptor, vasopressin V2 receptor, and leukotriene B4 receptor) with relatively high inhibition rates and acts on these ligands over a similar concentration range. It acts as a positive allosteric modulator of the GABAA receptor rather than an agonist. In periaqueductal grey neurons in rat midbrain slices, l-menthol was shown to prolong spontaneous GABAA receptor–mediated inhibitory current, most likely via a mechanism distinct from that of benzodiazepines. It acts on the dopamine D4 receptor and the dopamine transporter. l-menthol inhibits the [3H]-WIN35,428 binding, similar to GBR12909, suggesting that l-menthol inhibits the binding of dopamine to the dopamine transporter and leading to decreased dopamine uptake[1].
Biochem/physiol Actions
Taste at 25 ppm
Pharmacokinetics
Menthol is a covalent organic compound made synthetically or obtained from peppermint or other mint oils. Menthol induces a cooling sensation on the skin upon inhalation, oral ingestion, or topical application by stimulating the cold-sensitive receptors expressed on the skin, without actually causing a drop in the skin temperature.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. An eye irritant. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Metabolism
Purification Methods
Crystallise menthol from CHCl3,pet ether or EtOH/water. [Barrow & Atkinson J Chem Soc 638 1939, Beilstein 6 III 133, 6 IV 150.]
References
[1] Umezu T, et al. Identification of novel target molecules of l-menthol. Heliyon, 2021; 7: e07329.
Properties of L-Menthol
| Melting point: | 41-45 °C (lit.) |
| Boiling point: | 212 °C (lit.) |
| alpha | -51 º (589nm, c=10, EtOH) |
| Density | 0.89 g/mL at 25 °C (lit.) |
| vapor pressure | 0.8 mm Hg ( 20 °C) |
| refractive index | 1.46 |
| FEMA | 2665 | MENTHOL RACEMIC |
| Flash point: | 200 °F |
| storage temp. | Store below +30°C. |
| solubility | 490mg/l |
| form | Crystals or Crystalline Needles |
| pka | 15.30±0.60(Predicted) |
| Specific Gravity | 0.89 |
| color | Colorless to white |
| Odor | at 10.00 % in dipropylene glycol. peppermint cooling mentholic minty |
| optical activity | [α]22/D 49°, c = 10 in 95% ethanol |
| Water Solubility | insoluble |
| Merck | 14,5837 |
| BRN | 1902293 |
| Dielectric constant | 3.2(Ambient) |
| Stability: | Stable. |
| CAS DataBase Reference | 2216-51-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1R-(1«alpha»,2«beta»,5«alpha»)]-(2216-51-5) |
| EPA Substance Registry System | Levomenthol (2216-51-5) |
Safety information for L-Menthol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for L-Menthol
| InChIKey | NOOLISFMXDJSKH-KXUCPTDWSA-N |
L-Menthol manufacturer
Nectar Lifesciences Ltd
Swati Menthol and Allied Chemicals Ltd
Gloria Interchem Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(2S)-2-[[[(1,1-Dimethylethyl)diphenylsilyl]oxy]methyl]-1,3-oxathiolan-5-ol 5-Acetate](https://img.chemicalbook.in/CAS/20180601/GIF/202532-88-5.gif)
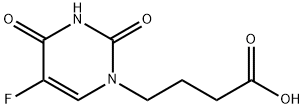
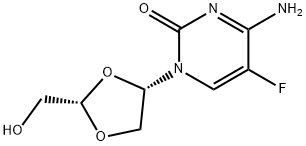
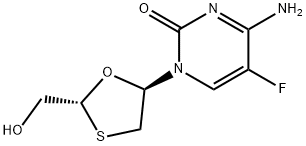
You may like
-
 2216-51-5 98%View Details
2216-51-5 98%View Details
2216-51-5 -
 MENTHOL CRYSTALS 99%View Details
MENTHOL CRYSTALS 99%View Details -
 Menthol Synthetic pure CAS 2216-51-5View Details
Menthol Synthetic pure CAS 2216-51-5View Details
2216-51-5 -
 L-Menthol CAS 2216-51-5View Details
L-Menthol CAS 2216-51-5View Details
2216-51-5 -
 Menthol, 99% CAS 2216-51-5View Details
Menthol, 99% CAS 2216-51-5View Details
2216-51-5 -
 MENTHOL CRYSTALS 99%View Details
MENTHOL CRYSTALS 99%View Details
2216-15-5 -
 Menthol Crystals 99% CAS 2216-51-5View Details
Menthol Crystals 99% CAS 2216-51-5View Details
2216-51-5 -
 (-)-Menthol CAS 2216-51-5View Details
(-)-Menthol CAS 2216-51-5View Details
2216-51-5