L-Homophe-OH
- CAS NO.:943-73-7
- Empirical Formula: C10H13NO2
- Molecular Weight: 179.22
- MDL number: MFCD00002619
- EINECS: 213-403-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-11 16:58:07
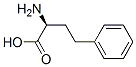
What is L-Homophe-OH?
Chemical properties
White Solid
What are the applications of Application
L-homophenylalanine is used almost exclusively as single stereoisomer in pharmaceutical drug production. L-homophenylalanine as a common building block, due to the presence of L-homophenylalanine moiety as the central pharmacophore unit. L-homophenylalanine is also an important chiral intermediate to synthesize a variety of novel pharmaceuticals including β-lactam antibiotics, acetylcholinesterase inhibitor and neutral endopeptidace (NEP) inhibitor which retained their status as major contributors to human health preservation. L-homophenylalanine acts as a precursor for the synthesis of NEP inhibitors, which complements the effects of ACE inhibitors when used simultaneously, potentiates the outcome in the management of hypertension and congestive heart failure[1].
The Uses of L-Homophe-OH
L-Homophenylalanine is used as a precursor in the pharmaceutical industry for the production of angiotensin-converting enzyme (ACE) and R-(-)-Homophenylalanin-ethylester. It is also involved in the synthesis of NEPA [(S,S)-N-(1-Ethoxycarbonyl-3-phenylpropyl)alanine], which is a key intermediate to prepare the ACE-inhibitors: enalapril, delapril, quinapril and ramipril. It acts as an anti-tumor reagent.
The Uses of L-Homophe-OH
Antitumor agent.
Definition
ChEBI: A non-proteinogenic L-alpha-amino acid that is an analogue of L-phenylalanine having a 2-phenylethyl rather than a benzyl side-chain.
Production Methods
Asymmetric reduction of prochiral ketone remains one of the most investigated methods to date for production of chiral L-homophenylalanine. One of the most established method for synthesizing L-homophenylalanine on a laboratory scale was carried out via enzyme-catalyzed asymmetric synthesis of keto acids. In this method, prochiral ketone was converted via reductive amination to enantiopure products with bulky side chains by addition of biocatalysts such as L-homophenylalanine dehydrogena. in the presence of cofactor. L-phenylalanine dehydrogenase is by and large preferred as it has mainly a catabolic function and accepts a wide variety of keto acids as substrates hence it is generally employed in the synthesis of L-homophenylalanine which carries very bulky side chains[1].
References
[1] Ahmad A, et al. Sustainable biocatalytic synthesis of L-homophenylalanine as pharmaceutical drug precursor. Biotechnology Advances, 2009; 27: 286-296.
Properties of L-Homophe-OH
| Melting point: | >300 °C(lit.) |
| alpha | 45 º (C=1, 3N HCl 19 ºC) |
| Boiling point: | 311.75°C (rough estimate) |
| Density | 1.1248 (rough estimate) |
| refractive index | 44 ° (C=1, 3mol/L HCl) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in dilute aqueous acid. |
| form | Solid |
| pka | 2.32±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1/C10H13NO2/c11-9(10(12)13)7-6-8-4-2-1-3-5-8/h1-5,9H,6-7,11H2,(H,12,13)/t9-/s3 |
| CAS DataBase Reference | 943-73-7(CAS DataBase Reference) |
Safety information for L-Homophe-OH
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-Homophe-OH
| InChIKey | JTTHKOPSMAVJFE-VIFPVBQESA-N |
| SMILES | C(O)(=O)[C@H](CCC1=CC=CC=C1)N |&1:3,r| |
L-Homophe-OH manufacturer
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 943-73-7 L-Homophenylalanine,97% 99%View Details
943-73-7 L-Homophenylalanine,97% 99%View Details
943-73-7 -
 L-Homophenylalanine CAS 943-73-7View Details
L-Homophenylalanine CAS 943-73-7View Details
943-73-7 -
 L-Homophenylalanine extrapure CAS 943-73-7View Details
L-Homophenylalanine extrapure CAS 943-73-7View Details
943-73-7 -
 L-Homophenylalanine 98% CAS 943-73-7View Details
L-Homophenylalanine 98% CAS 943-73-7View Details
943-73-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1