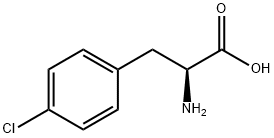
L-4-Cyanophenylalanine
- CAS NO.:167479-78-9
- Empirical Formula: C10H10N2O2
- Molecular Weight: 190.2
- MDL number: MFCD00270363
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-27 16:27:43
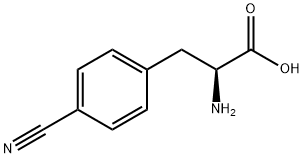
What is L-4-Cyanophenylalanine?
Description
p-Cyano-l-phenylalanine (pCNPhe) is an unnatural amino acid used in optical probes for imaging proteins. It was introduced in a 1994 world patent application by Richard T. Dean and co-inventors at Diatide (Londonderry, NH), who used it as a chelator for the radionuclide technetium-99m in a procedure for detecting blood clots (thrombi). The patent application was followed by a 1996 Journal of Medicinal Chemistry article.
Since that finding, pCNPhe showed its versatility in other applications. In a 2009 report, Daniel P. Raleigh, Isaac Carrico, and coauthors at the State University of New York at Stony Brook and Franklin & Marshall College (Lancaster, PA) used the molecule as a fluorescence, infrared (IR), and F?rster resonance energy transfer (FRET) probe to study protein folding, protein?membrane interactions, protein?protein interactions, and amyloid formation. They concluded that FRET, in particular, can be used directly to probe conformational changes in proteins.
In 2019, Megan C. Thielges and colleagues at Indiana University (Bloomington and Indianapolis) described the use of pCNPhe as an IR probe to characterize protein dynamics with high spatial and temporal resolution. They applied the probe at six sites in a Src1 homology 3 domain and found that their method revealed a wide range of microenvironments and distinct responses to ligand binding.
This past November, Paul S. Nerenberg, Christine M. Phillips-Piro, Scott H. Brewer, and collaborators at California State University, Los Angeles, and Franklin & Marshall once again demonstrated the utility of pCNPhe, this time as a “vibrational reporter” in the model system superfolder green fluorescent protein (sfGFP). They combined temperature-dependent IR spectroscopy, X-ray crystallography, and molecular dynamics simulations to investigate the local environment of the nitrile group in the protein. In one instance, they found that an interior site in sfGFP consists of three distinct local environments around the nitrile group, including nonspecific van der Waals interactions, hydrogen bonding to a structural water, and hydrogen bonding to a histidine side chain.
1. A proto-oncogene tyrosine–protein kinase.
The Uses of L-4-Cyanophenylalanine
4-Cyano-L-phenylalanine used as a intermediate in organic synthesis, pharmaceutical, chemical research and biochemical application.
Properties of L-4-Cyanophenylalanine
| Melting point: | not reported |
| Boiling point: | 394.1±37.0 °C(Predicted) |
| Density | 1.28±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | 100 g/l (est.) |
| appearance | white crystals or powder |
| form | Solid |
| pka | 2.13±0.10(Predicted) |
| Water Solubility | Slightly soluble in water. |
| CAS DataBase Reference | 167479-78-9(CAS DataBase Reference) |
Safety information for L-4-Cyanophenylalanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for L-4-Cyanophenylalanine
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 L-(S)-4-Cyanophenylalanine 96% CAS 167479-78-9View Details
L-(S)-4-Cyanophenylalanine 96% CAS 167479-78-9View Details
167479-78-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8