Isopropyl myristate
Synonym(s):Isopropyl myristate;IPM;Isopropylmyristate;Myristic acid isopropyl ester;Isopropyl tetradecanoate
- CAS NO.:110-27-0
- Empirical Formula: C17H34O2
- Molecular Weight: 270.45
- MDL number: MFCD00008982
- EINECS: 203-751-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41
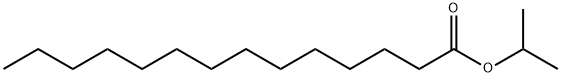
What is Isopropyl myristate ?
Absorption
Dermal absorption of isopropyl myristate is predicated to be 0.00020 mg/cm2/event, which is considered a very low absorption rate . In a study, topically applied isopropyl myristate was largely retained in the stratum corneum . It was not detected in the receptor fluid of flow-through diffusion cells in in-vitro skin permeation experiments using human epidermis (stratum corneum and viable epidermis) and dermis of varying thickness .
Toxicity
Readily available information regarding the pharmacokinetics of isopropyl myristate is not available .
Description
Isopropyl myristate is odorless when pure. May be synthesized by conventional esterification of isopropanol with myristic acid.
Chemical properties
colourless liquid of low viscosity
Chemical properties
Isopropyl myristate is a clear, colorless, practically odorless liquid of low viscosity that congeals at about 5°C. It consists of esters of propan-2-ol and saturated high molecular weight fatty acids, principally myristic acid.
Chemical properties
Pure isopropyl myristate is virtually odorless, very slightly fatty, but not rancid
Occurrence
Reported found in kumquat peel oil, papaya, starfruit, plum brandy, coriander seed and loquat
The Uses of Isopropyl myristate
Isopropyl myristate is an emollient in cosmetic and pharmaceutical bases.
The Uses of Isopropyl myristate
In cosmetic and topical medicinal Preparations where good absorption through the skin is desired. A jellied isopropyl myristate was marketed as Estergel (Merck & Co.) .
The Uses of Isopropyl myristate
isopropyl myristate is an emollient, moisturizer, binder, and skin softener that also assists in product penetration. An ester of myristic acid, it is naturally occurring in coconut oil and nutmeg. Although isopropyl myristate is generally considered comedogenic, some ingredient manufacturers clearly specify non-comedogenicity on their data sheets.
Background
Isopropyl myristate is a moisturizer with polar characteristics used in cosmetics and topical medical preparations to ameliorate the skin absorption. Isopropyl myristate has been largely studied and impulsed as a skin penetration enhancer.
At the moment the primary usage for which isopropyl myristate is formally indicated is as the active ingredient in a non-prescription pediculicide rinse .
Indications
The primary medical indication for which isopropyl myristate is formally used as an active ingredient in a patient care product is as a non-prescription pediculicide rinse .
Production Methods
Isopropyl myristate may be prepared either by the esterification of myristic acid with propan-2-ol or by the reaction of myristoyl chloride and propan-2-ol with the aid of a suitable dehydrochlorinating agent. A high-purity material is also commercially available, produced by enzymatic esterification at low temperature.
Preparation
By conventional esterification of isopropanol with myristic acid
Definition
ChEBI: Isopropyl tetradecanoate is a fatty acid ester.
brand name
Estergel (Merck).
General Description
Isopropyl myristate is an ester of isopropyl alcohol myristic acid. It is mainly used as a solubilizer, emulsifier and emollient in cosmetic and topical medicines. It also finds applications as a flavoring agent in the food industry.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical Applications
Isopropyl myristate is a nongreasy emollient that is absorbed readily
by the skin. It is used as a component of semisolid bases and as a
solvent for many substances applied topically. Applications in
topical pharmaceutical and cosmetic formulations include bath oils;
make-up; hair and nail care products; creams; lotions; lip products;
shaving products; skin lubricants; deodorants; otic suspensions; and
vaginal creams. For example, isopropyl myristate is a
self-emulsifying component of a proposed cold cream formula,
which is suitable for use as a vehicle for drugs or dermatological
actives; it is also used cosmetically in stable mixtures of water and
glycerol.
Isopropyl myristate is used as a penetration enhancer for
transdermal formulations, and has been used in conjunction with
therapeutic ultrasound and iontophoresis.It has been used in a
water-oil gel prolonged-release emulsion and in various microemulsions.
Such microemulsions may increase bioavailability in topical
and transdermal applications. Isopropyl myristate has also been
used in microspheres, and significantly increased the release of drug
from etoposide-loaded microspheres.
Isopropyl myristate is used in soft adhesives for pressuresensitive
adhesive tapes.
Contact allergens
Despite wide use in cosmetics, perfumes, and topical medicaments, isopropyl myristate is a very weak sen- sitizer and a mild irritant.
Biochem/physiol Actions
Isopropyl myristate (myristic acid isopropyl ester) is used to change the physicochemical characteristics of microsheres such as poly(lactic-co-glycolic acid) (PLGA) microspheres. Isopropyl myristate is used as a oil phase component in the formulaton of microemulsion systems.
Pharmacokinetics
Isopropyl myristate is an emollient vehicle that is effective at enhancing the penetration of other medical agents that may be incorporated into the vehicle as active agents . In one study, a 50:50 isopropanol-isopropyl myristate binary enhancer synergistically increased the transport of estradiol across a two-layer human epidermis in vitro .
Pharmacology
Isopropyl myristate is used in pharmaceutical preparations because it improves
solubility and increases absorption through the skin. External uses include a non-irritating iodine
preparation for disinfecting the skin (Powers & Rieger, 1963) and aerosol bactericidal preparations
for feminine hygiene use without irritation of the skin and mucous membranes (Geistlich, 1970;
Watson. 1969). Preparations for internal use include oral steroid formulations (Hirata, 1970) and
anaesthetic injection solutions (Davis, Pearce & Connor, 1972).
Veterinary medications containing isopropyl myristate include oral or parenteral compositions
for lungworm infections (N. V. Philips' Gloielampenfabrieken, 1964) and a spray formulation for
bovine udders to treat mastitis, combat infection and improve the general skin condition (Kraus,
1965). Isopropyl myristate has been found to be an effective repository vehicle for im injection
of penicillin in rabbits and for sc administration of oestrogens in ovariectomized rats (Platcow
& Voss, 1954).
In assays on human forearms, vasoconstrictor activity of ointment preparations containing 0025%
betamethasone 17-benzoate in white soft paraffin was increased by the presence of isopropyl myristate
(Pepler, Woodford & Morrison, 1971). Donovan, Ohmart & Stoklosa (1954) noted that the good
solvent properties of isopropyl myristate might increase the therapeutic activity of formulations
by the apparent alteration in particle size of the active ingredients, so that further evaluation and
clinical study would be necessary before its use in extemporaneous compounding could be recommended.
Studies in which the antifungal activity of paraben esters solubilized by surfactants was
decreased by isopropyl myristate (Matsumoto & Aoki, 1962) indicate that the effectiveness of medicinal
substances may be influenced by the presence of surfactants and oily ingredients such as
isopropyl myristate.
Side Effects
Thrapecylate myristate is a medicine used to treat head lice infestations in adults and children 4 years of age and older. Common side effects include skin irritation, rash, and contact dermatitis.
Safety
Isopropyl myristate is widely used in cosmetics and topical
pharmaceutical formulations, and is generally regarded as a
nontoxic and nonirritant material.
LD50 (mouse, oral): 49.7 g/kg
LD50 (rabbit, skin): 5 g/kg
Synthesis
Isopropyl myristate is synthesized by conventional esterification of iso-Propanol with Myristic acid.
Metabolism
Any isopropyl myristate that is absorbed is likely to be hydrolyzed to its component compounds of isopropylalcohol and myristic acid .
Metabolism
Higher molecular weight aliphatic esters are thought to be readily hydrolysed to the corresponding alcohols and acids which are then metabolized; isopropyl myristate is undoubtedly hydrolysed to normal metabolic products (Fassett, 1963). When myristic acid (as the ethyl ester) was fed to dogs,
Storage
Isopropyl myristate is resistant to oxidation and hydrolysis, and does not become rancid. It should be stored in a well-closed container in a cool, dry place and protected from light.
Incompatibilities
When isopropyl myristate comes into contact with rubber, there is a drop in viscosity with concomitant swelling and partial dissolution of the rubber; contact with plastics, e.g. nylon and polyethylene, results in swelling. Isopropyl myristate is incompatible with hard paraffin, producing a granular mixture. It is also incompatible with strong oxidizing agents.
Regulatory Status
Included in the FDA Inactive Ingredients Database (otic, topical, transdermal, and vaginal preparations). Used in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Isopropyl myristate
| Melting point: | ~3 °C (lit.) |
| Boiling point: | 193 °C/20 mmHg (lit.) |
| Density | 0.85 g/mL at 25 °C (lit.) |
| vapor pressure | <1 hPa (20 °C) |
| refractive index | n |
| FEMA | 3556 | ISOPROPYL MYRISTATE |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | <0.05mg/l |
| form | Liquid |
| color | Clear |
| Specific Gravity | 0.855 (20/4℃) |
| Odor | odorless |
| Water Solubility | Miscible with alcohol. Immiscible with water and glycerol. |
| Merck | 14,5215 |
| JECFA Number | 311 |
| BRN | 1781127 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 110-27-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Isopropyl Myristate(110-27-0) |
| EPA Substance Registry System | Isopropyl myristate (110-27-0) |
Safety information for Isopropyl myristate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P321:Specific treatment (see … on this label). P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Isopropyl myristate
| InChIKey | AXISYYRBXTVTFY-UHFFFAOYSA-N |
Isopropyl myristate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 ISO PROPYL MYRISTATE 99%View Details
ISO PROPYL MYRISTATE 99%View Details -
 Isopropyl Myristate CAS 110-27-0View Details
Isopropyl Myristate CAS 110-27-0View Details
110-27-0 -
 Isopropyl Myristate pure CAS 110-27-0View Details
Isopropyl Myristate pure CAS 110-27-0View Details
110-27-0 -
 Isopropyl Myristate extrapure AR (0.2um filtered) CAS 110-27-0View Details
Isopropyl Myristate extrapure AR (0.2um filtered) CAS 110-27-0View Details
110-27-0 -
 Isopropyl myristate 98% (GC) CAS 110-27-0View Details
Isopropyl myristate 98% (GC) CAS 110-27-0View Details
110-27-0 -
 Isopropyl Myristate, 150 KgView Details
Isopropyl Myristate, 150 KgView Details
110-27-0 -
 Isopropyl Myristate ChemicalView Details
Isopropyl Myristate ChemicalView Details
110-27-0 -
 Isopropyl Myristate, 125 mlView Details
Isopropyl Myristate, 125 mlView Details
110-27-0
