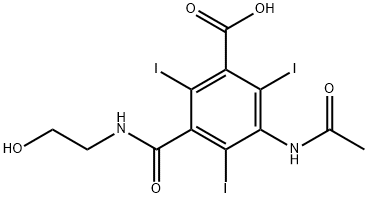
IOXITALAMIC ACID
- CAS NO.:28179-44-4
- Empirical Formula: C12H11I3N2O5
- Molecular Weight: 643.94
- MDL number: MFCD00867942
- EINECS: 248-887-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is IOXITALAMIC ACID?
Absorption
When administered ioxitalamate is not absorbed in the GI tract. In the case of presence of an intestinal perforation, ioxitalamate is completely absorbed. When administered intravascularly, ioxitalamate is rapidly distributed in the interstitial space and intravascular compartment.
Toxicity
Ioxitalamate is reported to induce acute kidney injury. Preclinical studies showed that it does not present a teratogenic potential as well as a lack of effect on fertility. Ioxitalamate overdose may increase the risk of nephropathy and cardiovascular disorders.
Chemical properties
White Solid
Originator
Oxilan,Cook Imaging Corporation
The Uses of IOXITALAMIC ACID
A substituted 2,4,6-triiodobenzoic acid, an excellent contrast media for ventriculography, radiculography, lumbar myelography and x-rays of the cardiovascular system.
Background
Ioxitalamate is an ionic iodinated contrast medium. It is a first-generation contrast media formed by an ionic monomer with a high osmolarity of 1500-1800 mOsm/kg. Ioxitalamic acid in the salt forms of sodium and meglumine was developed by Liebel-Flarshem Canada Inc and approved by Health Canada in 1995. Until the last review in 2015, this drug is still available in the market.
Indications
Ioxitalamate in both of its available forms is indicated for exploration of the digestive tract by tomodensitometry or by regular gastroduodenal radiography. Its use is restrained to the cases in which the administration of barium sulfate is not recommended or contraindicated. The intravascular administration of ioxitalamate is contraindicated as it may present significant side effects.
What are the applications of Application
Ioxitalamic Acid is a contrast medium
Definition
ChEBI: An organoiodine compound that is 2,4,6-triiodobenzoic acid substituted by an acetylamino group at position 3 and a (2-hydroxyethyl)carbamoyl group at position 5. It is used as a contrast medium.
Manufacturing Process
3-Methoxycarboxyl-5-nitrobenzoic acid (25 g) was hydrogenated in methanol
(500 ml) using palladium oxide on charcoal (2.5 g 10%) at atmospheric
pressure. When the exothermic reaction was completed the catalyst was
fluttered off. After cooling the solution at -20°C for 2.5 h, 12.7 g of 3-amino-
5-methoxycarbonylbenzoic acid was isolated. An additional 6.5 g of it was
isolated by concentrating the mother liquor.
The 3-amino-5-methoxycarbonylbenzoic acid (12.0 g) was suspended in water
(280 ml), dissolved by addition of concentrated hydrochloric acid (7.1 ml) and
glacial acetic acid (28.5 ml). At 60°-70°C NaICl2 solution (73 ml, 58.7 g
ICl/100 ml) was added dropwise while stirring in the course of about 3 h. The
reaction mixture was heated at 80°-90°C for additional 3 h while stirring.
After cooling to room temperature the mother liquor was decanted and the
residue dissolved as ammonium salt in water (80 ml). The ammonium salt
was precipitated by adding ammonium chloride (2.4 g) and cooling to 0°C.
The ammonium salt was filtered off and dissolved in water (140 ml),
charcoaled twice at 80°C and the acid was precipitated at room temperature
by addition of hydrochloric acid and was filtered off. The crude product was
dissolved in ethyl acetate (100 ml) and the solution was washed 3 times with
hydrochloric acid (2 N). By evaporating the solvent, 19 g of 3-amino-5-
methoxycarbonyl-2,4,6-triiodobenzoic acid was isolated. Melting point 170°-
176°C.
A mixture of 3-amino-5-methoxycarbonyl-2,4,6-triiodobenzoic acid (198 g)
and thionyl chloride (400 ml) was heated while stirring at 70°C for 16 h. The
solid material dissolved slowly. Thionyl chloride was evaporated in vacuo, the
residue dissolved in chloroform (1000 ml), the solution washed with water (80
ml each), twice with saturated sodium bicarbonate, then 5 times with 2 N
sodium hydroxide solution and finally with water to neutral. The solution was
dried with CaCl2 filtered and evaporated to dryness. The 3-amino-5-
methoxycarbonyl-2,4,6-triiodobenzoyl chloride was dried at 50°C in vacuo.
Yield: 203.0 g. Melting point 55°-60°C.
To the 3-amino-5-methoxycarbonyl-2,4,6-triiodobenzoyl chloride (53.0 g) was
added acetic anhydride (106 ml). After stirring at room temperature for 20
min then insoluble material was filtered off (3-4 g). To the filtrate was added
concentrated sulfuric acid (0.3 ml) whereby a yellowish product started to
precipitate. The temperature reached about 50°C. The 3-acetamido-5-
methoxycarbonyl-2,4,6-triiodobenzoyl chloride was isolated after storing in
refrigerator overnight. Yield: 39.0 g. Melting point 210°-215°C.
The 3-acetamido-5-methoxycarbonyl-2,4,6-triiodobenzoyl chloride was
dissolved in a mixture of dioxan and dimethylformamide. In the course of 2 h
this solution was added dropwise to a solution of ethanolamine and
triethylamine in dioxan. The stirring was continued. A sticky precipitate was
filtered off. The filtrate was evaporated to dryness in vacuo. The residue was
triturated with aqueous sodium bicarbonate, filtered off and mixed with first
fraction. The combined solids were then suspended in aqueous sodium
bicarbonate filtered off washed with water and dried in vacuo to give methyl
5-acetamido-2,4,6-triiodo-(N-β-hydroxyethyl)-isophthalamate.
The methyl 5-acetamido-2,4,6-triiodo-(N-β-hydroxyethyl)-isophthalamate was
mixed with fresh distilled ethanolamine and stirred. The excess ethanolamine
was removed in vacuo at 50°-60°C. The residue was dissolved in water, and
charcoaled at pH 5.5. The crude product was precipitated with hydrochloric
acid (pH 0.5) and filtered after stirring at 0°C. 5-Acetamido-2,4,6-triiodo-(N-
β-hydroxyethyl)isophthalamic acid was suspended in ethanol and dissolved by
addition of concentrated ammonia. The ammonium salt started to precipitate
in the course and was isolated after stirring. The salt was dissolved in water,
filtered and the acid was precipitated with hydrochloric acid (pH 0.5). After
stirring the product was filtered off and dried in vacuo.
Therapeutic Function
Diagnostic aid
Pharmacokinetics
Ioxitalamate presents a very large osmolality which is related to the presence of renal toxicity, vasodilatation, bradycardia and pulmonary hypertension. This large osmolality allows ioxitalamate to move slowly in the bowel allowing for analysis for later follow excretion in the feces.
Metabolism
The rapid clearance suggests that ioxitalamate is not metabolized in the body.
Properties of IOXITALAMIC ACID
| Melting point: | 253-255?C |
| Boiling point: | 582.8±50.0 °C(Predicted) |
| Density | 2.519±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| pka | 0.85±0.10(Predicted) |
| color | Off-White to Pale Brown |
Safety information for IOXITALAMIC ACID
Computed Descriptors for IOXITALAMIC ACID
IOXITALAMIC ACID manufacturer
Blue Jet Healthcare Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Ioxitalamic acid >98%View Details
Ioxitalamic acid >98%View Details -
![3-(Acetylamino)-5-{[(2-hydroxyethyl)amino]carbonyl}-2,4,6-triiodobenzoic acid CAS](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(Acetylamino)-5-{[(2-hydroxyethyl)amino]carbonyl}-2,4,6-triiodobenzoic acid CASView Details
3-(Acetylamino)-5-{[(2-hydroxyethyl)amino]carbonyl}-2,4,6-triiodobenzoic acid CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1