INSULIN
Synonym(s):Bovine insulin;Cell culture grade insulin;Insulin from bovine pancreas
- CAS NO.:11070-73-8
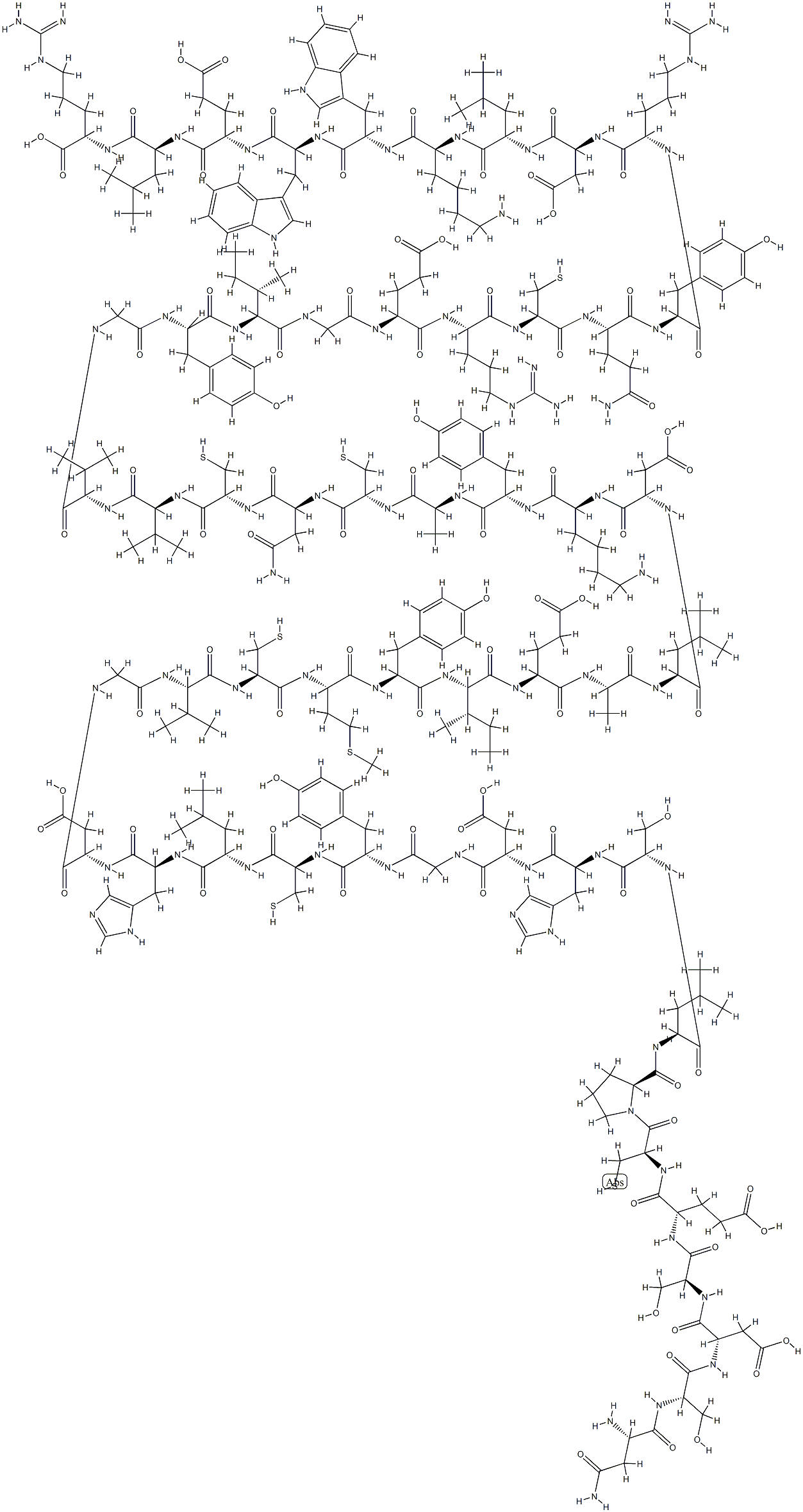
- Empirical Formula: C254H377N65O75S6
- Molecular Weight: 5733.49
- MDL number: MFCD02321930
- EINECS: 234-291-2
- Update Date: 2024-11-11 14:08:43
What is INSULIN?
Chemical properties
white solid
Chemical properties
Insulin is a relatively simple protein consisting of 51 amino acids arranged as two polypeptide chains, an α- chain and β-chain, connected by disulfide bonds; the latter are necessary to maintain tertiary structure and biological activity. Although the amino acid sequence and composition of animal insulins may differ slightly from those of human insulin, their biological actions are similar. Alteration of specific amino acid residues within the insulin molecule yields novel derivatives that vary in their pharmacokinetics and binding affinity for the insulin receptor. Some insulin analogues display mitogenic properties in addition to their metabolic effects.
The Uses of INSULIN
Insulin is a peptide hormone regulating the metabolism of carbohydrates and fats. It is used in the treatment of those suffering from the diabetes type I and II metabolic disorders. Regulates the absorption of glucose from the blood to skeletal muscles and fat storage.
The Uses of INSULIN
Antidiabetic.
The Uses of INSULIN
Recommended for use in cell culture applications at 0.5 to 1 mL per liter of medium.
Definition
A polypeptide hormone having a molecular weight of 5733. It is formed in the islets of Langerhans located in the pancreas and was so named for this reason. Insulin is composed of 16 amino acids arranged in a coiled chain and crosslinked in several places
Indications
More than a century has passed since von Mering and Minkowski first demonstrated that pancreatectomized dogs exhibited signs and symptoms characteristic of diabetes mellitus. Shortly thereafter, Banting and Best used pancreatic extracts to reverse these symptoms in diabetic patients, thus providing a basis for establishing a cause-and-effect relationship between insulin deficiency and diabetes. Insulin was subsequently isolated, crystallized, and eventually synthesized in the laboratory. Insulin replacement therapy has been widely used in the clinical management of diabetes mellitus for more than 70 years. In 1982, recombinant DNA (rDNA) derived human insulin was first produced and is now widely used instead of insulin derived from beef or pork. More recently, insulin analogues have been produced that modulate the activity and rate of insulin action.
Hazard
Overdosage can be fatal.
Biochem/physiol Actions
Two-chain polypeptide hormone produced by the β-cells of pancreatic islets. Its molecular weight is ~5800 Da. The α and β chains are joined by two interchain disulfide bonds. The α chain contains an intrachain disulfide bond. Insulin regulates the cellular uptake, utilization, and storage of glucose, amino acids, and fatty acids and inhibits the breakdown of glycogen, protein, and fat.
Pharmacology
Insulin is usually administered subcutaneously. Depending
on the type of insulin being administered,
the rate of insulin absorption can be modulated by altering the polymerization of the insulin molecule (e.g.,
monomers, dimers, or hexamers). Intramuscular injections
of insulin are used less often because absorption is
more rapid. Being a polypeptide hormone, insulin is
readily inactivated if administered orally. In emergencies,
such as severe diabetic ketoacidosis, insulin can be
given intravenously. Clinical studies are examining the
efficacy and safety of inhaled insulin, which may be
promising for some patients.
Once insulin enters the circulation, its plasma halflife
is less than 10 minutes. Hepatic insulinases destroy
approximately 50% of circulating insulin, with the remainder
degraded by circulating proteases. Therefore,
only a relatively small amount of the total endogenous
insulin secreted ever reaches the peripheral tissues.
Although a number of tissues accumulate small amounts
of insulin, the liver and kidney are the principal sites of
hormone uptake and degradation. Insulin metabolism is
accomplished both through the actions of an insulinspecific
protease found in the cytosol of many tissues
and by the reductive cleavage of the insulin disulfide
bonds by glutathione–insulin transhydrogenase. In the
kidney, insulin that undergoes glomerular filtration is almost
completely reabsorbed and metabolized within the
proximal convoluted tubules of the nephron.
Clinical Use
According to the DCCT and the UK Prospective Diabetes study, insulin and/or insulin analogues are the standard treatment for type 1, gestational, and some type 2 diabetes.
Side Effects
Insulin Overdose and Diabetic Coma The most common and serious reaction to insulin therapy is hypoglycemia. It is important that patients with diabetes, especially those receiving insulin therapy, be able to recognize the signs and symptoms of hypoglycemia. Symptoms of hypoglycemia may be evident with a plasma glucose level at 60 to 80 mg/dL. Severe hypoglycemia can lead to convulsions and coma. Patients that vigorously attempt to achieve euglycemia to avoid various vascular complications risk increased frequency of hypoglycemic episodes. In the DCCT, the incidence of severe hypoglycemic reactions was threefold higher in the intensive insulin therapy group than in the conventional therapy group.
Metabolism
Insulin degradation occurs primarily in the liver and kidney. Of that which is secreted from the pancreatic islet cells, 50% reaches the liver via the portal vein and undergoes disulfide bond cleavage catalyzed by glutathione insulin transhydrogenase (insulinase). This is followed by proteolytic degradation before entry into the general circulation. Insulin is filtered by the renal glomeruli and can then be reabsorbed or degraded by the tubules. At the tissue level, insulin degradation occurs to a limited extent at the cell surface.
Properties of INSULIN
| storage temp. | -20°C |
| solubility | acidified water, pH 2.0: 2 mg/mL |
| form | solution |
| color | White to off-white |
| Merck | 13,5003 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Keep refrigerated at -20 C |
| CAS DataBase Reference | 11070-73-8 |
Safety information for INSULIN
Computed Descriptors for INSULIN
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Insulin, from bovine pancreas CAS 11070-73-8View Details
Insulin, from bovine pancreas CAS 11070-73-8View Details
11070-73-8 -
 Insulin, ≥ 25 USP units/mg (HPLC), powder CAS 11070-73-8View Details
Insulin, ≥ 25 USP units/mg (HPLC), powder CAS 11070-73-8View Details
11070-73-8 -
 Insulin from bovine pancreas CAS 11070-73-8View Details
Insulin from bovine pancreas CAS 11070-73-8View Details
11070-73-8 -
 Insulin from bovine pancreas CAS 11070-73-8View Details
Insulin from bovine pancreas CAS 11070-73-8View Details
11070-73-8 -
 ProteoMass™ Insulin MALDI-MS Standard CAS 11070-73-8View Details
ProteoMass™ Insulin MALDI-MS Standard CAS 11070-73-8View Details
11070-73-8 -
 Insulin solution from bovine pancreas CAS 11070-73-8View Details
Insulin solution from bovine pancreas CAS 11070-73-8View Details
11070-73-8 -
 Insulin from bovine pancreas solution CAS 11070-73-8View Details
Insulin from bovine pancreas solution CAS 11070-73-8View Details
11070-73-8 -
 Insulin from bovine pancreas CAS 11070-73-8View Details
Insulin from bovine pancreas CAS 11070-73-8View Details
11070-73-8