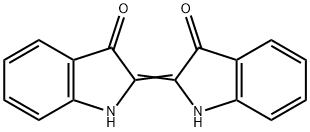
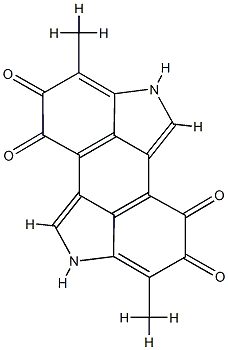
indigoidine
- CAS NO.:2435-59-8
- Empirical Formula: C10H8N4O4
- Molecular Weight: 248.19
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-11-23 09:42:38
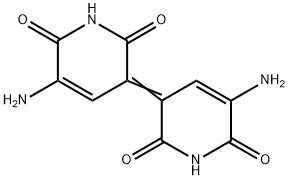
What is indigoidine?
Description
Indigoidine is a blue-violet organic pigment related to the Azaquinones group and synthesized by bacterial strains. It is biosynthesized by bacterial species such as Streptomyces chromofuscus,E. coli,and Corynebacterium insidiosum (Gangulyet al. 2019). It is used as a food colorant in cereal, baking, and ice-cream industries.
The Uses of indigoidine
Indigoidine natural blue dye is a promising alternative to the synthetic dyes used to colored jeans, leather, food, beverages, cosmetics and paper. The blue color of the indigoidine compound is bright and sustainable. As a natural dye, it has promising health benefits as an antioxidant and antimicrobial actor. Because of a new purification process patented with the compound, indigoidine natural blue dye is safe to use in food and drinks.
Definition
ChEBI: Indigoidine is a member of the class of pyridone that is a dimeric blue pigment biosynthesised from L-glutamine. It has a role as a bacterial metabolite and a biological pigment. It is a pyridone, a ring assembly, a dicarboximide and an enamine. It derives from a L-glutamine.
Definition
Pigment characterization:
Identification of interrupted genes in the nonpigmented mutants suggested that the blue compound produced by Y4I was indigoidine, a bicyclic 3,3′-bipyridyl molecule synthesized by the cyclization of two glutamine molecules. The absorbance spectrum of the purified compound in DMSO has a peak at 612 nm, which has been previously reported as the absorbance maximum of indigoidine. Profiles of the Y4I variant show that absorbance at this wavelength for the clpA::Tn5 mutant is approximately five times higher than that of the wild-type strain[1].
Biosynthesis
Indigoidine is a water-insoluble blue pigment that was first isolated from phytopathogenic Erwinia as a powerful radical scavenger that enables phytopathogens to tolerate oxidative stress, organic peroxides, and superoxides during the plant defense response due to its structure of carbon–carbon double bonds conjugated with a carbonyl group. This bacterial pigment shows a bright blue color similar to that of indigo. Several different strains are reported to produce it. Indigoidine is assembled from two units of L-glutamine by a nonribosomal peptide synthetase (e.g. IndC from Erwinia chrysanthemi and Streptomyces aureofaciens CCM 3239, BpsA from Streptomyces lavendulae and Sc-indC from Streptomyces chromofuscus ATCC 49982) (Figure 1.7b). Recently, an indigoidine biosynthetic gene cluster was located in the genome of S. chromofuscus ATCC 49982. The gene cluster is silent and consists of five open reading frames, called orf1, Sc-indC, Sc-indA, Sc-indB, and orf2. Sc-IndC was functionally characterized as an indigoidine synthase through heterologous expression of the enzyme in both Streptomyces coelicolor CH999 and E. coli BAP1. The titer of indigoidine in E. coli BAP1 was reported to be 2.78 g/L under optimized conditions. Its production was dramatically increased (by 41.4%/3.93 g/L) when Sc-IndB was co-expressed with it in E. coli BAP1 (Yu et al. 2013). In order to further improve production, a glutamine synthetase gene was amplified from E. coli and co-expressed with Sc-indC and Sc-indB in E. coli BAP1. At 2.5 mM (NH4)2HPO4, the titer can reach 7.08±0.11 g/L (Xu et al. 2015). This provides a green, efficient production process for this promising blue dye.
Biological Activity
Research has suggested that indigoidine protects D. dadantii by neutralizing reactive oxygen species generated by the plant during bacterial infection. This was the only reported biological role for indigoidine [1].
Solubility in water
Indigoidine is found in two redox states, an oxidized blue form which is insoluble in water and a reduced colorless form which is water soluble and historically referred to as leucoindigoidine[1].
References
[1] W Nathan Cude. “Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I.” Applied and Environmental Microbiology 78 14 (2012): 4771–80.
Properties of indigoidine
| Boiling point: | 285.6±40.0 °C(Predicted) |
| Density | 1.637±0.06 g/cm3(Predicted) |
| pka | 5.88±0.40(Predicted) |
Safety information for indigoidine
Computed Descriptors for indigoidine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4