Ferroglycine Sulfate
- CAS NO.:17169-60-7
- Empirical Formula: C2H4FeNO6S
- Molecular Weight: 225.96
- EINECS: 241-221-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-27 14:11:44
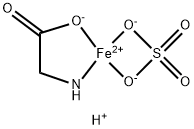
What is Ferroglycine Sulfate?
Description
Iron can be supplemented orally using various chemical formulations. Among them, the one based on the ferrous glycine sulfate (known as ferroglycine sulfate complex; FeGS) is very favourable due to a reduced presence of gastrointestinal side effects compared to standard preparations based on iron fumarate. FeGS is a FeII complex with the amino acid glycine. The 1:2 metal-to-ligand ratio restricts reaction with dietary inhibitors of iron absorption, neutralizes the valence of the ferrous FeII, and protects gastrointestinal surfaces from irritation by metal cations. Therefore, this structure makes FeGS an ideal fortifier, especially for foods with a high content of absorption inhibitors such as phytate[1].
Originator
Ferronord,Cooper,US,1956
Definition
Iron(II) gluconate (a ferrous gluconate) is used to treat hypochromic anemia. The real problem of iron therapy is not the theoretical utilization of iron, the reticulocyte response, or even the daily increase of hemoglobin. These are important only as they indicate the return of the patients' blood to normal in a reasonably short time without undue inconvenience. Most patients suffering from hypochromic anemia respond well to most forms of iron when administered in adequate dosage. For patients who cannot tolerate the usual iron compounds, it is essential to have a medication that is effective and causes minimum disturbance. Ferrous gluconate is such a medicament.
Manufacturing Process
10.0 g of ferrous sulfate and 2.7 g of glycine are thoroughly mixed and carefully heated under nitrogen to 70°C. Reaction occurs rapidly, and the complex compound is obtained as soon as the color turns uniformly lightbrown. After cooling to 20°C, 12.7 g of ferrous sulfate-glycine complex are obtained, which contains 100 mg Fe++-ion sper 0.63 g.
Therapeutic Function
Hematinic
Flammability and Explosibility
Not classified
structure and hydrogen bonding
The coordination polyhedron around the metal atom can be considered a strongly distorted octahedron with elongated axial edges. Figure 2 and Table 1 give the details of the coordination sphere around the metal cation. The equatorial oxygen atoms are separated from the metal centre at distances expected for a crystal structure of this kind [FeII–O bond lengths of 2.15(1) ?, 2.11(1) ?, 2.12(1) ? and 2.15(1) ?], where the axial oxygen atoms are found at unusually long distances [2.39(1) ? and 2.37(1) ?]. These lengths indicate very weak bonding, and it can be argued whether FeII–O contacts of ca. 2.4 ? are considered a classical chemical bond. Therefore, the coordination sphere around the FeII cation can even be considered square-planar.![hydrogen (glycinato-N,O)[sulphato(2-)-O,O']ferrate(1-) hydrogen (glycinato-N,O)[sulphato(2-)-O,O']ferrate(1-)](/NewsImg/2024-06-11/6385369401487353212031090.png)
References
[1] R. Dinnebier, B. Hinrichsen, T. Run?evski. “Crystal Structure of the Dietary Supplement Ferrous Glycine Sulfate.” Zeitschrift für anorganische und allgemeine Chemie 37 3 1 (2016): 306–310.
Properties of Ferroglycine Sulfate
| vapor pressure | 0Pa at 25℃ |
| Water Solubility | 480g/L at 20℃ |
| InChI | InChI=1S/C2H4NO2.Fe.H2O4S/c3-1-2(4)5;;1-5(2,3)4/h3H,1H2,(H,4,5);;(H2,1,2,3,4)/q-1;+3;/p-2 |
Safety information for Ferroglycine Sulfate
Computed Descriptors for Ferroglycine Sulfate
| InChIKey | QDNVKBLOCGKQEB-UHFFFAOYSA-L |
| SMILES | O=S1([O-][Fe+2]2(NCC(=O)[O-]2)[O-]1)=O.[H+] |
Abamectin manufacturer
ShanPar Industries Pvt Ltd
Global Calcium Pvt Ltd
Ferro chem Industries
Aspen Biopharma Labs Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidYou may like
-
 17169-60-7 Ferroglycine sulfate 99%View Details
17169-60-7 Ferroglycine sulfate 99%View Details
17169-60-7 -
 17169-60-7 99%View Details
17169-60-7 99%View Details
17169-60-7 -
 Ferroglycine sulfate 99%View Details
Ferroglycine sulfate 99%View Details
17169-60-7 -
 Ferroglycine sulfate 17169-60-7 98%View Details
Ferroglycine sulfate 17169-60-7 98%View Details
17169-60-7 -
 17169-60-7 98%View Details
17169-60-7 98%View Details
17169-60-7 -
 Ferrous glycine sulphate CAS 17169-60-7View Details
Ferrous glycine sulphate CAS 17169-60-7View Details
17169-60-7 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4