Hydrocortisone IMpurity I
- CAS NO.:103795-84-2
- Empirical Formula: C21H30O6
- Molecular Weight: 378.46
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
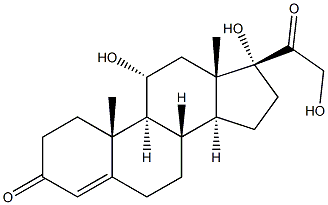
What is Hydrocortisone IMpurity I?
The Uses of Hydrocortisone IMpurity I
14α-Hydroxycortisol (Hydrocortisone EP Impurity I) is an impurity of Hydrocortisone (H714615), a topical corticosteroid (1), but more specifically a glucocorticoid, produced by the zona fasciculata of the adrenal gland (2). Cortisol is released in response to stress and a low level of blood glucocorticoids. Its primary functions are to increase blood sugar through gluconeogenesis; suppress the immune system; and aid in fat, protein and carbohydrate metabolism.
Properties of Hydrocortisone IMpurity I
| Melting point: | 241-242 °C |
| Boiling point: | 602.3±55.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| pka | 12.10±0.70(Predicted) |
Safety information for Hydrocortisone IMpurity I
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Hydrocortisone IMpurity I
Hydrocortisone IMpurity I manufacturer
Allmpus Laboratories Pvt Ltd
1Y
Phone:+91-9167862134
Whatsapp: +91-9167862134
product: 103795-84-2 Hydrocortisone EP Impurity I 95.8
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 103795-84-2 Hydrocortisone EP Impurity I 95.8View Details
103795-84-2 Hydrocortisone EP Impurity I 95.8View Details
103795-84-2 -
 103795-84-2 HYDROCORTISONE EP IMPURITY I 97View Details
103795-84-2 HYDROCORTISONE EP IMPURITY I 97View Details
103795-84-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.