HEXYLTRICHLOROSILANE
Synonym(s):Hexyltrichlorosilane
- CAS NO.:928-65-4
- Empirical Formula: C6H13Cl3Si
- Molecular Weight: 219.61
- MDL number: MFCD00013606
- EINECS: 213-178-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
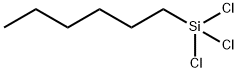
What is HEXYLTRICHLOROSILANE?
Description
Hexyl trichlorosilane is a colorless liquidwhich fumes in moist air. Molecular weight= 219.63;Boiling point= 191-192℃; Flash point = 85℃. HazardIdentification (basedon NFPA-704 M Rating System):Health 3,Flammability 2,Reactivity 2W. Reacts withwater.
Chemical properties
Colorless liquid; sharp penetrating odor. Fumes strongly in moist air.
The Uses of HEXYLTRICHLOROSILANE
Chemical intermediate.
General Description
HEXYLTRICHLOROSILANE is a colorless liquid with a pungent odor. HEXYLTRICHLOROSILANE is decomposed by moist air or water to hydrochloric acid with evolution of heat. HEXYLTRICHLOROSILANE is corrosive to metals and tissue. HEXYLTRICHLOROSILANE is used as a chemical intermediate.
Reactivity Profile
Chlorosilanes, such as HEXYLTRICHLOROSILANE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Hazard
Toxic by ingestion and inhalation, strong irritant. Combustible.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Potential Exposure
Used in the manufacture of other sili-con chemicals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Do not make an unconsciousperson vomit. Medical observation is recommended for24 - -48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corti-costeroid spray.
Storage
(1) Color Code- -White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location. (2)Color Code- -Blue: Health Hazard/Poison: Store in a securepoison location. Prior to working with this chemical youshould be trained on its proper handling and storage. Store intightly closed containers in a cool, well-ventilated area awayfrom water and steam. Hexyl trichlorosilane can give off cor-rosive hydrogen chloride gas on contact with water, steam, ormoisture. W here possible, automatically pump liquid fromdrums or other storage containers to process containers.
Shipping
Hexyl trichlorosilane requires a shipping label of“CORROSIVE.”It falls in Hazard Class 8 and PackingGroup II.
Incompatibilities
Reacts violently with strong oxidizers.Reacts violently with water, moisture, and steam producingchlorine and hydrogen chloride. Attacks active metals (e.g.,aluminum and magnesium). Chlorosilanes on contact withammoniaforms a self- igniting product.
Properties of HEXYLTRICHLOROSILANE
| Boiling point: | 191-192 °C(lit.) |
| Density | 1.107 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 185 °F |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 1.107 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1739071 |
| CAS DataBase Reference | 928-65-4(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, trichlorohexyl- (928-65-4) |
Safety information for HEXYLTRICHLOROSILANE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for HEXYLTRICHLOROSILANE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Trichlorohexylsilane CAS 928-65-4View Details
Trichlorohexylsilane CAS 928-65-4View Details
928-65-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
