Hexafluoroethane
Synonym(s):CFC-116;Perfluoroethane
- CAS NO.:76-16-4
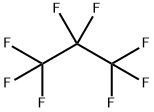
- Empirical Formula: C2F6
- Molecular Weight: 138.01
- MDL number: MFCD00000428
- EINECS: 200-939-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-27 13:18:55
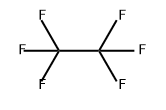
What is Hexafluoroethane?
Description
Hexafluoroethane is a colorless and oodorlessgas or liquid under pressure. Molecular weight = 138.02;Boiling point= 一78℃. Hazard Identification (based onNFPA-704 M Rating System): Health 1, Flammability 0,Reactivity 0. Insoluble in water.
Chemical properties
Hexafluoroethane is a colorless and odorless gas, or liquid under pressure. asphyxiant in high concentrations. Gas density is heavier than air. Hexafluoroethane is chemically inert and has a very high global warming potential (GWP = 11,100; Greenhouse gas protocol, Fifth Assessment Report AR5). It is an etchant and chamber cleaning agent.
The Uses of Hexafluoroethane
Hexafluoroethane is used as an important component in some refrigeration mixtures, and as a reactive ion etching gas in semiconductor materials processing applications. It can be used for selective etching of metal silicides and oxides versus their metal substrates and also for etching of silicon dioxide over silicon.
In semiconductor applications, for example in reactive ion etching, hexafluoroethane with a fluorine to carbon ratio of 3:1 offers a unique source of highly reactive trifluoromethyl radicals without accompanying hydrogen atoms.As a result, semiconductor material plasma etching processes employing hexafluoroethane are relatively aggressive with respect to fluorine radical formation.
Definition
ChEBI: Hexafluoroethane is a fluoroalkane and a fluorocarbon. It has a role as a refrigerant.
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 3007, 1955 DOI: 10.1021/ja01616a025
General Description
HEXAFLUOROETHANE is a colorless, odorless gas. HEXAFLUOROETHANE is relatively inert. The mixture is nonflammable and nontoxic, though asphyxiation may occur because of displacement of oxygen. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
Reactivity Profile
HEXAFLUOROETHANE is chemically inert in many situations, but can react violently with strong reducing agents such as the very active metals and the active metals. Can react with strong oxidizing agents or weaker oxidizing agents under extremes of temperature.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Potential Exposure
It is used as a coolant, in dielectric fluids; as a propellant and refrigerant.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irri gate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothingand wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Iffrostbite has occured, seek medical attention immediately;do NOT rub the affected areas or flush them with water. Inorder to prevent further tissue damage, do NOT attempt toremove frozen clothing from frostbitten areas. If frostbitehasNOT occurred, immediately and thoroughly washcontaminated skin with soap and water.
Storage
Color Code- Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-venti lated area away from metals,including aluminum, zinc, and beryllium, and from openflames or temperatures above 52℃. Procedures for thehandling, use, and storage of cylinders should be in compli-ance with OSHA 1910.101 and 1910.169, as with therecommendations of the Compressed Gas Association.
Shipping
UN2193 Hexafluoroethane or Refrigerant gas R-116, Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed gas.
Purification Methods
Purify it for pyrolysis studies by passing through a copper vessel containing CoF3 at ca 270o, and hold for 3hours in a bottle with a heated (1300o) platinum wire. It is then fractionally distilled. [Steunenberg & Cady J Am Chem Soc 74 4165 1962, Beilstein 1 IV 132.]
Incompatibilities
Active metals. Keep away from heat and sunlight.
Waste Disposal
Return refillable compressed gas cylinders to supplier.
Properties of Hexafluoroethane
| Melting point: | −100 °C(lit.) |
| Boiling point: | −78 °C(lit.) |
| Density | 1,607 g/cm3 |
| vapor density | 4.7 (vs air) |
| vapor pressure | 430 psi ( 21 °C) |
| refractive index | 1.2060 |
| CAS DataBase Reference | 76-16-4(CAS DataBase Reference) |
| EPA Substance Registry System | Hexafluoroethane (76-16-4) |
Safety information for Hexafluoroethane
| Signal word | Warning |
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H280:Gases under pressure |
| Precautionary Statement Codes |
P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for Hexafluoroethane
| InChIKey | WMIYKQLTONQJES-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
