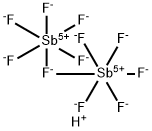
Fluoroantimonic acid
Synonym(s):Fluoroantimonic acid;Hexafluoroantimonic acid;Hexafluorostibonic acid;Monohydrogen hexafluoroantimonate
- CAS NO.:16950-06-4
- Empirical Formula: F6HSb
- Molecular Weight: 236.76
- MDL number: MFCD00011327
- EINECS: 241-023-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
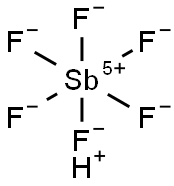
What is Fluoroantimonic acid?
Description
Fluoroantimonic acid (HSbF6), the strongest known superacid, is made by combining HF with SbF5. It can be as much as 2 × 1019?times stronger than 100% H2SO4. However, superacids are all equally strong in aqueous solution; other solvents must be used to observe their effects.
Chemical properties
superacid; moisture sensitive [KIR78] [ALD94]
Strongest superacid known
mixture of HF and SbF5 (1:1 is strongest)
Protonate even hydrocarbons to afford carbocations and H2.
2×1019 (20 quintillion) times stronger than 100% sulfuric acid.
Ho values of -31.3
The Uses of Fluoroantimonic acid
Hydrogen hexafluoroantimonate(V) is an excellent medium for the preparation of alkylidene oxonium salts.
Preparation
Fluoroantimonic acid is produced by reacting hydrogen fluoride (HF) with antimony pentafluoride (SbF5).
SbF5+HF→SbF6+HF
General Description
HF/SbF5 may be synthesized by mixing hydrogen fluoride and antimony fluoride.1 Fluoroantimonic acid (HF/SbF5), is a combination of SbF5 and HF. It is the known strongest liquid superacid.
Properties of Fluoroantimonic acid
| Melting point: | 20°C |
| Density | 2.885 g/mL at 25 °C(lit.) |
| vapor pressure | 14 mm Hg ( 18 °C) |
| form | liquid |
| color | yellow to colorless |
| Water Solubility | It is fully miscible with water, soluble in SO2ClF, SO2. |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| CAS DataBase Reference | 16950-06-4(CAS DataBase Reference) |
| EPA Substance Registry System | Antimonate(1-), hexafluoro-, hydrogen, (OC-6-11)- (16950-06-4) |
Safety information for Fluoroantimonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fluoroantimonic acid
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![PHENYL-[M-(2-HYDROXYTETRADECYLOXY)PHENYL]IODONIUM HEXAFLUOROANTIMONATE](https://img.chemicalbook.in/CAS/GIF/139301-14-7.gif)

You may like
-
 Hydrogen hexafluoroantimonate(V) CAS 16950-06-4View Details
Hydrogen hexafluoroantimonate(V) CAS 16950-06-4View Details
16950-06-4 -
 Fluoroantimonic acid CAS 16950-06-4View Details
Fluoroantimonic acid CAS 16950-06-4View Details
16950-06-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1