Guanidine hydrochloride
Synonym(s):Guanidine hydrochloride;Guanidinium chloride;Guanidium chloride;Guanidinum hydrochloride, Carbamimidoylazanium chloride;Aminoformamidine hydrochloride
- CAS NO.:50-01-1
- Empirical Formula: CH5N3.ClH
- Molecular Weight: 95.53
- MDL number: MFCD00013026
- EINECS: 200-002-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
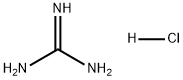
What is Guanidine hydrochloride?
Chemical properties
Guanidine hydrochloride, a strong organic base, appears as white crystal powder or yellowish lumps that are almost insoluble in acetone, benzene, and ether. In physiological pH, it exists primarily as guanidium ions. It is a natural byproduct of protein metabolism found in urine and is commonly used in protein denaturation in laboratory research.
The Uses of Guanidine hydrochloride
Guanidine hydrochloride is used for purification of proteins and nucleic acids. It can be used as a medicine, organic synthesis intermediate and is used in dyes. It acts as an intermediate in the preparation of sulfadiazine, which is an important raw material for sulfamethyldiazine, sulfamethazine and folic acid. It is utilized in the synthesis of 2-amino-pyrimidine, 2- amino -6- methyl-pyrimidine and 2-amino -4,6 - dimethyl-pyrimidine. Also used in RNA isolation to dissociate nucleoproteins and inhibit RNase.
The Uses of Guanidine hydrochloride
Guanidine has a good bacterial and fungicidal efficacy when used in low concentration and can also be used as disinfectant.
What are the applications of Application
Guanidine Hydrochloride is a widely used and powerful protein denaturant used in the purification of proteins and nucleic acids
Definition
ChEBI: Guanidine Hydrochloride is an aminocarboxamidine, the parent compound of the guanidines. It is a strong organic base existing primarily as guanidium ions at physiological pH. It is found in the urine as a normal product of protein metabolism.
What are the applications of Application
Guanidine hydrochloride can be used as pharmaceuticals, pesticides, dyes and other organic synthesis intermediates. It can be used to synthesize 2-aminopyrimidine, 2-amino-6-methylpyrimidine, 2-amino-4,6-dimethylpyrimidine, and is used for the manufacture of sulfadiazine, sulfamethazine, sulfamethazine and other sulfa drugs. Intermediate. Guanidine hydrochloride (or guanidine nitrate) reacts with ethyl cyanoacetate to form 2,4-diamino-6-hydroxypyrimidine, which is used to synthesize the anti-anemia drug folic acid. It can also be used as an antistatic agent for synthetic fibers. Can also be used for protein denaturants. As a strong denaturant in experiments for extracting total cellular RNA[3]. The guanidine hydrochloride solution can dissolve the protein, leading to the destruction of the cell structure, the destruction of the secondary structure of the nucleoprotein, and the dissociation from the nucleic acid. In addition, the RNase can be inactivated by reducing agents such as guanidine hydrochloride.
Preparation
The process for preparing guanidine hydrochloride is as follows:
Step 1: Fritting - Combine dicyandiamide and ammonium chloride in a reactor with a 1:1.27 weight ratio and carry out a frit reaction at 170-230°C to obtain crude guanidine hydrochloride.
Step 2: Dissolving - Dissolve the crude guanidine hydrochloride in water at a 1:1 ratio at normal temperature. Then, add ammonium salt alkaloid in an amount determined by the ammonium salt content in the crude guanidine hydrochloride.
Step 3: Filtering - Remove raw materials and reaction by-products with filtration.
Step 4: Dehydrating - Dehydrate the filtered ground mother liquor at a high temperature.
Step 5: Crystallizing - Cool the supersaturated solution, concentrate, and crystallize to obtain high-purity guanidine hydrochloride.
Step 6: Repeat steps 2-5 and add dicyandiamide in the reactor with the guanidine hydrochloride to carry out frit reaction at 170-230°C for 3-4 hours to obtain even higher purity guanidine hydrochloride. This step can be repeated several times if needed.
Step 7: To obtain higher purity guanidine hydrochloride, subject the guanidine hydrochloride obtained in step 5 to steps 2-5 again, with the water ratio in step 2 adjusted to 1.5:1. This step can be repeated numerous times as required.
General Description
Guanidine hydrochloride (GdnHCl) is a well-known protein and enzyme denaturant. It is a small molecule and hydroscopic in nature. It is used to synthesis medicine, pesticide, dye and other organic compound, is the important material to make sulfanilamide medicine and folacin, and can be used as anti-static agent of compound fibre.
Hazard
Toxic by ingestion, evolves hydrogen chloride fumes on heating.
Biochem/physiol Actions
Guanidine hydrochloride (GdnHCl) is involved in conformational changes and loss of enzyme activity due to inactivation of the enzyme. It is involved in the loss of activity of alkaline phosphatase (ALPase) enzyme in Haliotis diversicolor. GdnHCl functions by hampering the activity of heat shock protein 104 (Hsp104) adenosine triphosphatase (ATPase) in vivo. It can also inactivate enzymes like papain and aminocyclase. Higher concentrations of GdnHCl leads to viral inactivation of herpes simplex virus 1 (HSV-1).
Purification Methods
Crystallise the hydrochloride from hot methanol by chilling to about -10o, with vigorous stirring. The fine crystals are filtered through fritted glass, washed with cold (-10o) methanol, dried at 50o under vacuum for 5hours. (The product is purer than that obtained by crystallisation at room temperature from methanol by adding large amounts of diethyl ether.) [Kolthoff et al. J Am Chem Soc 79 5102 1957, Beilstein 3 H 86, 3 II 71, 3 III 160, 3 IV 150.]
Properties of Guanidine hydrochloride
| Melting point: | 180-185 °C(lit.) |
| Density | 1.18 g/mL at 25 °C(lit.) |
| refractive index | n |
| storage temp. | room temp |
| solubility | H2O: 6 M, clear, colorless |
| form | Crystals |
| color | White to slightly yellow |
| Odor | Odorless |
| PH | 4.5-5.5 (100g/l, H2O, 20℃) |
| PH Range | 4.5 - 6.0 at 573 g/l at 25 °C |
| Water Solubility | 2280 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.040 λ: 280 nm Amax: 0.025 |
| Merck | 14,4562 |
| BRN | 3591990 |
| Stability: | Stable. Hygroscopic. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-01-1(CAS DataBase Reference) |
| EPA Substance Registry System | Guanidine monohydrochloride (50-01-1) |
Safety information for Guanidine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Guanidine hydrochloride
| InChIKey | PJJJBBJSCAKJQF-UHFFFAOYSA-N |
Guanidine hydrochloride manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![[(2,6-DICHLOROPHENYL)ACETYL]GUANIDINE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/29110-48-3.gif)
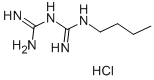
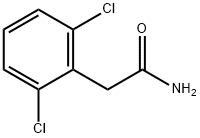
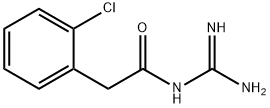
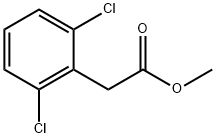
You may like
-
 GUANIDINE HYDROCHLORIDE 99%View Details
GUANIDINE HYDROCHLORIDE 99%View Details -
 Guanidine hydrochloride CAS 50-01-1View Details
Guanidine hydrochloride CAS 50-01-1View Details
50-01-1 -
 Guanidine Hydrochloride CAS 50-01-1View Details
Guanidine Hydrochloride CAS 50-01-1View Details
50-01-1 -
 Guanidine Hydrochloride (GHC) for molecular biology CAS 50-01-1View Details
Guanidine Hydrochloride (GHC) for molecular biology CAS 50-01-1View Details
50-01-1 -
 Guanidine hydrochloride CAS 50-01-1View Details
Guanidine hydrochloride CAS 50-01-1View Details
50-01-1 -
 Technical Grade Crystals Guanidine HydrochlorideView Details
Technical Grade Crystals Guanidine HydrochlorideView Details
113-00-8 -
 Guanidine Hydrochloride Cas 50011, Technical Grade, DrumView Details
Guanidine Hydrochloride Cas 50011, Technical Grade, DrumView Details
50-01-1 -
 Guanidine Hydrochloride (GHC) (CAS Number: 50-01-1)View Details
Guanidine Hydrochloride (GHC) (CAS Number: 50-01-1)View Details
50-01-1
