Glutamic acid
Synonym(s):(S)-2-Aminopentanedioic acid;L -Glutamic acid;Glu
- CAS NO.:6899-05-4
- Empirical Formula: C5H9NO4
- Molecular Weight: 147.13
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-28 13:53:20
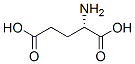
What is Glutamic acid?
Description
Glutamic acid (abbreviated as Glu or E) is one of the 20-22 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates. In neuro science, glutamate is an important neuro transmitter that plays a key role in long-term potentiation and is important for learning and memory.
Chemical properties
The side chain carboxylic acid functional group has a pKa of 4.1 and therefore exists almost entirely in its negatively charged deprotonated carboxylate form at pH values greater than 4.1; therefore, it is negatively charged at physiological pH ranging from 7.35 to 7.45.
History
Although they occur naturally in many foods, the flavour contributions made by glutamic acid and other amino acids were only scientifically identified early in the twentieth century. The substance was discovered and identified in the year 1866, by the German chemist Karl Heinrich Leopold Ritthausen who treated wheat gluten (for which it was named) with sulfuric acid. In 1908 Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavour he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavour umami. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate.
The Uses of Glutamic acid
glutamic acid is a moisture binder and an anti-oxidant. glutamic acid is an amino acid manufactured by means of fermentation, generally from a vegetable protein.
The Uses of Glutamic acid
Glutamic Acid is an amino acid that is a white crystalline powder of slight solubility in water. the salt is monosodium glutamate (msg) which functions as a flavor enhancer in meats. it also is a nutrient, dietary supplement, and salt substitute.
The Uses of Glutamic acid
Metabolism
Glutamate is a key compound in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase.
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the vertebrate nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the NMDA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, glutamate is involved in cognitive functions like learning and memory in the brain. The form of plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain. Glutamate works not only as a point-to-point transmitter but also through spill-over synaptic crosstalk between synapses in which summation of glutamate released from a neighboring synapse creates extrasynaptic signaling / volume transmission.
Glutamate transporters are found in neuronal and glial membranes . They rapidly remove glutamate from the extracellular space. In brain injury or disease, they can work in reverse, and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via NMDA receptor channels, leading to neuronal damage and eventual cell death, and is called excitotoxicity.
Brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophila brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitization . A gene expressed in glial cells actively transports glutamate into the extracellular space, while, in the nucleus accumbens-stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels. This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
Flavor enhancer
Glutamic acid, being a constituent of protein, is present in every food that contains protein, but it can only be tasted when it is present in an unbound form. Significant amounts of free glutamic acid are present in a wide variety of foods, including cheese and soy sauce, and is responsible for umami, one of the five basic tastes of the human sense of taste. Glutamic acid is often used as a food additive and flavour enhancer in the form of its salt, known as monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, and kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. Thirty to 35 % of the protein in wheat is glutamic acid. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass.
Plant growth
Auxigro is a plant growth preparation that contains 30 % glutamic acid.
NMR spectroscopy
In recent years, there has been much research into the use of RDCs in NMR spectroscopy. A glutamic acid derivative, poly-γ- benzyl-L-glutamate (PBLG), is often used as an alignment medium to control the scale of the dipolar interactions observed.
Production Methods
China-based Fufeng Group Limited is the largest producer of glutamic acid in the world, with capacity increasing to 300,000 tons at the end of 2006 , Meihua is the second - largest Chinese producer. Together, the top-five producers have roughly 50 % share in China. Chinese demand is roughly 1.1 million tons per year.
Definition
ChEBI: Glutamic acid is an alpha-amino acid that is glutaric acid bearing a single amino substituent at position 2. It has a role as a fundamental metabolite. It is an alpha-amino acid and a polar amino acid. It contains a 2-carboxyethyl group. It is a conjugate acid of a glutamate(1-).
Synthesis Reference(s)
Canadian Journal of Chemistry, 34, p. 1440, 1956 DOI: 10.1139/v56-184
Pharmacology
The drug phencyclidine (more commonly known as PCP) antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, dextromethorphan and ketamine also have strong dissociative and hallucinogenic effects. Glutamate does not easily pass the blood brain barrier, but , instead, is transported by a high-affinity transport system . It can also be converted into glutamine.
Properties of Glutamic acid
| CAS DataBase Reference | 6899-05-4 |
Safety information for Glutamic acid
Computed Descriptors for Glutamic acid
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1