GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
Synonym(s):Prepro-65-77-Apelin
- CAS NO.:217082-58-1
- Empirical Formula: C69H111N23O16S
- Molecular Weight: 1550.83
- MDL number: MFCD02094627
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-27 11:49:37
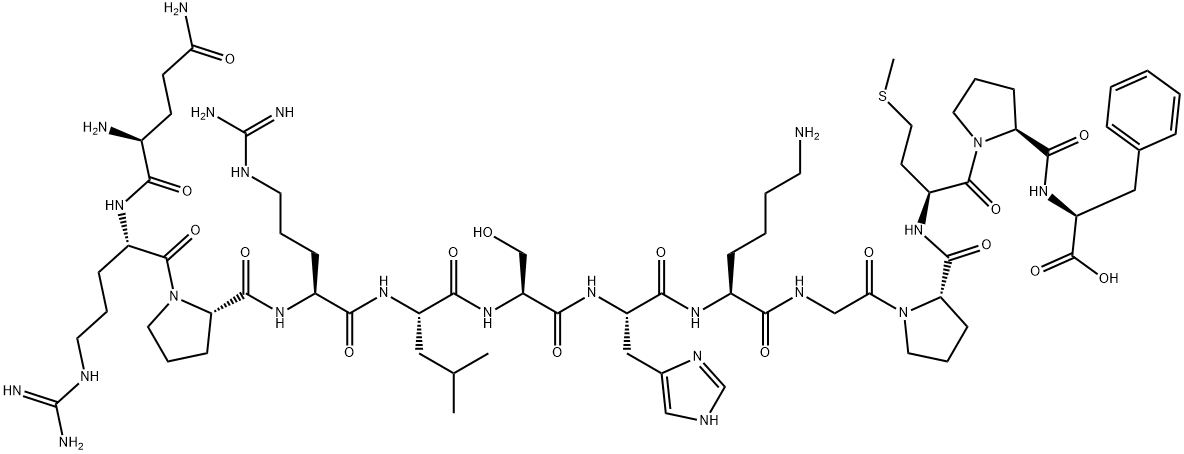
What is GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE?
The Uses of GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
The apelin gene encodes a pre-proprotein that is processed to generate bioactive peptides consisting of 36, 17, or 13 amino acids (apelin-36, apelin-17, and apelin-13, respectively). Apelin-13 is the endogenous ligand of the APJ receptor, activating this G protein-coupled receptor with an EC50 value of 0.37 nM (the EC50 values for apelin-17 and apelin-36 are 2.5 and 20 nM, respectively). It acts primarily in the periphery and central nervous system, playing important roles in regulating cardiovascular function, fluid homeostasis, hypertension, and insulin sensitivity. Unlike apelin-36, apelin-13 poorly blocks the entry of human immunodeficiency virus into cells.
The Uses of GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
An endogenous ligand of Apelin Receptor (APLNR/APJ receptor) that plays important roles in regulating fluid homeostasis, cardiovascular function, and insulin sensitivity.
The Uses of GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
Apelin-13 trifluoroacetate salt has been used to determine whether apelin promotes progression of diabetic nephropathy (DN) by inducing podocyte dysfunction. It has also been used to examine the effects of apelin on myocardial infarction (MI) injury and underlying mechanisms.
What are the applications of Application
Apelin-13 trifluoroacetate salt is an endogenous ligand of G-protein coupled receptor APJ
What are the applications of Application
Apelin-13 is a 13-amino acid polypeptide and endogenous ligand of the G-protein coupled receptor APJ.
Definition
ChEBI: Apelin-13 is a 13 amino acid oligopeptide which is the ligand for the apelin receptor (also known as the APJ receptor). It exhibits hypotensive and neuroprotective effects, and may be a potential prognostic biomarker for acute ischemic stroke and multiple sclerosis. It has a role as an antihypertensive agent, a biomarker, an autophagy inhibitor, a neuroprotective agent and a human metabolite. It is a conjugate base of an apelin-13(3+).
General Description
Apelin is a regulatory peptide and a ligand of the APJ receptor. It is a member of the G protein-coupled receptor family. Apelin and APJ are broadly dispersed in the body.
Biological Activity
apelin-13 is an endogenous ligand of the apj receptor.the apelin receptor apj, one of a group of g-proteincoupled receptors (gpcr), have recently been paired with their cognate peptide ligands using ‘‘reverse pharmacology’’, and functional evidence suggests a role for this receptor in the regulation of cardiovascular function, fluid homeostasis, and as a coreceptor for hiv infection.
Biochem/physiol Actions
Apelin plays physiological roles in the cytoprotection of many internal organs. It is used to treat insulin resistance and hypertension, but it may also can act as a negative factor. Apelin also regulates cardiovascular diseases.
in vitro
apelin-13 was identified as an endogenous ligand of the apj receptor, which could activate this g protein-coupled receptor with an ec50 value of 0.37 nm. in addition, the ec50 values for apelin-17 and apelin-36 have been found to be 2.5 and 20 nm, respectively [1].
in vivo
in a previous study, urethane anaesthetised, paralysed and ventilated male sd rats were used to investigate the action of apelin-13 directly microinjected into the nucleus tractus solitarius (nts) and the rostral ventrolateral medulla (rvlm) on arterial pressure and phrenic nerve activity. results showed that apelin-13 microinjections into the nts led to either apnea or decreased phrenic nerve discharge amplitude by up to 30%. in the rvlm, apelin-13 caused increase in phrenic nerve discharge amplitude depending on the exact site of injection [2].
References
[1] lee, d. k.,cheng, r.,nguyen, t., et al. characterization of apelin, the ligand for the apj receptor. journal of neurochemistry 74, 34-41 (2000).
[2] seyedabadi m, goodchild ak, pilowsky pm. site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. auton neurosci. 2002 oct 31;101(1-2):32-8.
[3] a g japp, n l cruden, g barnes, n van gemeren, j mathews, j adamson, n r johnston, m a denvir, i l megson, a d flapan, d e newby. acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. circulation 2010 april 27, 121 (16): 1818-27.
Properties of GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
| Density | 1.50±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | Soluble in PBS (pH 7.2) at approximately 10 mg/ml. |
| form | Lyophilized powder |
| pka | 3.56±0.10(Predicted) |
Safety information for GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
Computed Descriptors for GLN-ARG-PRO-ARG-LEU-SER-HIS-LYS-GLY-PRO-MET-PRO-PHE
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Apelin-13 trifluoroacetate salt CASView Details
Apelin-13 trifluoroacetate salt CASView Details -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4