GHRELIN (HUMAN)
- CAS NO.:258279-04-8
- Empirical Formula: C149H249N47O42
- Molecular Weight: 3370.86
- MDL number: MFCD07366494
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
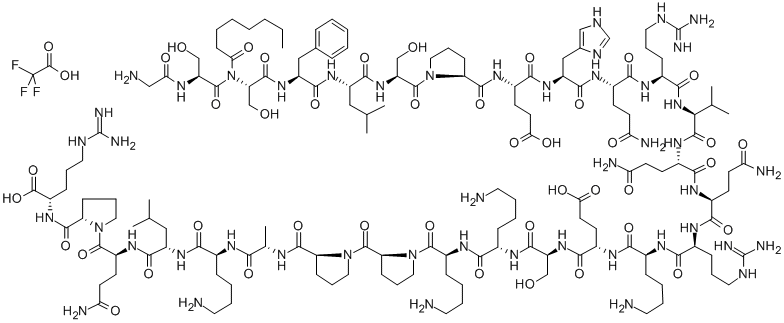
What is GHRELIN (HUMAN)?
The Uses of GHRELIN (HUMAN)
The GHS-R (growth hormone secretagogue receptor) potently stimulates growth hormone release from the pituitary gland in vivo and in vitro. It stimulates food intake and transduces signals to hypothalamic regulatory nuclei that control energy homeostasis. Ghrelin (human) is an activator of GHS-R1a.
The Uses of GHRELIN (HUMAN)
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, is synthesized principally in the stomach. It stimulates food intake and transduces signals to hypothalamic regulatory nuclei that control energy homeostasis. It is a peptide of 28 amino acids, in which the serine 3 residue is n-octanoylated, which is necessary for biological activity.
What are the applications of Application
Ghrelin (human) is an endogenous peptide GHS-R agonist
Biological Activity
ghrelin is a specific endogenous ligand for the ghs receptor which can stimulate strong increase in circulating gh levels both in vitro and in vivo in a dose-dependent manner, human acylated ghrelin is the major active form that can cross the blood-brain barrier, and has an effection on the activity of arcute neurones with a much stronger affinity (ic50, 0.3×10-9m) than ghsr antagonist (d-lys3)-ghrp-6 (ic50, 0.9×10-6m)[1].ghrelin has effects in stimulating appetite, increasing secretion of gh and hypo-catabolism, improvement of gastrointestinal motility, increasing cardiac output, improvement of pulmonary function and anti-inflammatory effect.clinical applications of ghrelin in total gastrectomy, esophagectomy and neoadjuvant chemotherapy can stimulate food intake, improve guality of life scores and minimize adverse events. recently, ghrelin as a effective approach in the management of anorexia nervosa[2],cachexia[3], bw[4] and appetite loss[5], systemic inflammatory response[6]and cancer[7]. in conclusion, ghrelin is a promising candidate for treating catabolic states and enhancing immune function in cachexia or acquired immunodeficiency syndrome, as well as for treating eating disorders such as obesity and anorexia nervosa.
storage
Store at -20°C
References
1. m. traebert,* t. riediger,† s. whitebread,* e. scharrer† and h. a. schmid*. ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. journal of neuroendocrinology, 2002, vol. 14, 580–5862. hotta m, ohwada r, akamizu t, shibasaki t, takano k, kangawa k. ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. endocr j. 2009;56:1119–28.3. nagaya n, itoh t, murakami s, oya h, uematsu m, miyatake k, et al. treatment of cachexia with ghrelin in patients with copd. chest. 2005;128:1187–93.4. adachi s, takiguchi s, okada k, yamamoto k, yamasaki m, miyata h, et al. effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase ii study. gastroenterology. 2010;138:1312–20.5. hiura y, takiguchi s, yamamoto k, kurokawa y, yamasaki m, nakajima k, et al. fall in plasma ghrelin concentrations after cisplatin-based chemotherapy in esophageal cancer patients. int j clin oncol. 2012;17:316–23.6. cheyuo c, jacob a, wang p. ghrelin-mediated sympathoinhibition and suppression of inflammation in sepsis. am j physiol endocrinol metab. 2012;302:e265–72.7. yoshida n, watanabe m, baba y, iwagami s, ishimoto t, iwatsuki m, et al. risk factors for pulmonary complications after esophagectomy for esophageal cancer. surg today. 2014;44:526–32.
Properties of GHRELIN (HUMAN)
| Density | 1.49±0.1 g/cm3(Predicted) |
| storage temp. | -15°C |
| solubility | Soluble in H2O |
| form | Solid |
| Water Solubility | Soluble in water, and 5% acetic acid. |
Safety information for GHRELIN (HUMAN)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for GHRELIN (HUMAN)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran





![[TYR4]GHRELIN, HUMAN](https://img.chemicalbook.in/)
You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0