GERMANIUM TETRABROMIDE
Synonym(s):Germanium tetrabromide
- CAS NO.:13450-92-5
- Empirical Formula: Br4Ge
- Molecular Weight: 392.26
- MDL number: MFCD00016115
- EINECS: 236-612-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
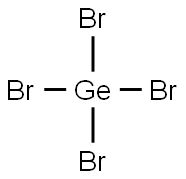
What is GERMANIUM TETRABROMIDE?
Chemical properties
solid ingot in glass with 99.999% purity; white crystal(s); enthalpy of vaporization 41.4 kJ/mol; can be prepared readily by reacting Ge with Br2 or with GeO2 and HBr solutions [KIR78] [STR93] [CER91] [CRC10]
The Uses of GERMANIUM TETRABROMIDE
It is used as a chemical agent, as a laboratory chemical & for manufacturing of substances.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits very toxic fumes of Br-. See also GERMANIUM COMPOUNDS.
Purification Methods
Purify it by simple distillation or fractionation depending on purity. It is soluble in EtOH, CHCl3, *C6H6 and Et2O. It fumes in moist air and is readily hydrolysed by water. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 718 1963]. LACHRYMATORY.
Properties of GERMANIUM TETRABROMIDE
| Melting point: | 26.1 °C(lit.) |
| Boiling point: | 186.5 °C(lit.) |
| Density | 3.13 g/mL at 25 °C(lit.) |
| refractive index | 1.6269 |
| Flash point: | 186.5°C |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | reacts with H2O |
| form | Low Melting Solid/Liquid, Ampouled Under Argon |
| color | White to gray |
| Specific Gravity | 3.132 |
| Water Solubility | It reacts in water. |
| Sensitive | Moisture Sensitive |
| Stability: | Stable. Incompatible with water, strong oxidizing agents. |
| CAS DataBase Reference | 13450-92-5(CAS DataBase Reference) |
| EPA Substance Registry System | Germane, tetrabromo- (13450-92-5) |
Safety information for GERMANIUM TETRABROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for GERMANIUM TETRABROMIDE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
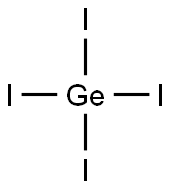
You may like
-
 Germanium(IV) bromide CAS 13450-92-5View Details
Germanium(IV) bromide CAS 13450-92-5View Details
13450-92-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.