Gamma Butyrolactone
Synonym(s):γ-Butyrolactone;γ-Hydroxybutyric acid lactone;4-Hydroxybutyric acid lactone;gamma-Butyrolactone;GBL
- CAS NO.:96-48-0
- Empirical Formula: C4H6O2
- Molecular Weight: 86.09
- MDL number: MFCD00005386
- EINECS: 202-509-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
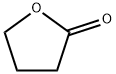
What is Gamma Butyrolactone?
Description
Dihydro-2(3H)-furanone. An endogenous neuroregulator made from gamma-amino butyrate and the precursor of gamma hydroxybutyrate. It causes selective increase of brain dopamine by inhibiting its release from nerve terminals. The compound has sedative properties at low doses and produces surgical anesthesia at high doses. It is also used as an industrial solvent and precursor.
Chemical properties
γ-Butyrolactone is oily, colorless, clear liquid. It has a faint, sweet, aromatic, slightly buttery odor.

γ-Butyrolactone is a lactone. It is hydrolyzed under basic conditions, for example in a sodium hydroxide solution into sodium gamma-hydroxybutyrate, the sodium salt of gamma-hydroxybutyric acid. Under acidic conditions it forms an equilibrium mixture of both compounds. These compounds then may go on to form a polymer.
Occurrence
Reported found as a constituent in coffee aroma; a volatile flavor component in roasted filberts as well. Also reported found in tomato, potato, soybeans, beans, vinegar, mushrooms, roasted chicken, beef, cider, beer, wine, scallops and clams.
The Uses of Gamma Butyrolactone
For the introduction of 3-carboxypropyl side chain. Used as a component of electrolyte solutions in batteries and capacitors.
The Uses of Gamma Butyrolactone
r-butyrolactone is one kind of important fine chemicalintermediate, simultaneously also is one kind of performance fine highboiling point solvent, ideal antioxidant, plasticizer,extracting agent, absorbent, dispersing agent, solid stain, Coagulation Reagent.
The Uses of Gamma Butyrolactone
Intermediate in the synthesis of polyvinylpyrrolidone, DL-methionine, piperidine, phenylbutyric acid, thiobutyric acids. Solvent for polyacrylonitrile, cellulose acetate, methyl methacrylate polymers, polystyrene. Constituent of paint removers, textile aids, drilling oils.
Definition
ChEBI: A butan-4-olide that is tetrahydrofuran substituted by an oxo group at position 2.
Preparation
γ-Butyrolactone is produced by gas phase 1,4-butanediol under the action of Cu catalyst to produce product γ-butyrolactone and by-product hydrogen. Crude γ-Butyrolactone is purified to remove light and heavy components, with a purity of more than 99.5%, as an export product. At the same time, the by-product hydrogen is sent to other hydrogenation processes for recycling after removing CO and CO2 through the methanation process.
Aroma threshold values
Detection: 20 to 50 ppm
Taste threshold values
Taste characteristic at 75 ppm: milky, creamy with fruity peach-like afternotes.
General Description
Clear colorless oily liquid with a pleasant odor.
Air & Water Reactions
Hygroscopic. Soluble in water.
Reactivity Profile
gamma-Butyrolactone can react with oxidizing materials, inorganic acids and bases, alcohols and amines. Rapidly hydrolyzed by bases and slowly hydrolyzed by acids. gamma-Butyrolactone is volatile with steam. . The combination of the lactone, butanol, 2,4-dichlorophenol, and sodium hydroxide in the attempted synthesis of 2,4-dichlorophenoxybutyric acid caused a thermal runaway reaction that eventually exploded, [CISHC Chem. Safety Summ., 1977, 48, 3].
Hazard
Toxic by ingestion. Questionable carcino- gen.
Fire Hazard
gamma-Butyrolactone is combustible.
Biochem/physiol Actions
Precursor of γ-hydroxybutyric acid (GHB). It blocks dopamine release by blocking impulse flow in dopaminergic neurons. Pretreatment with γ-butyrolactone allows detection of autoreceptor-induced dopamine release.
Safety Profile
Moderately toxic by ingestion, intravenous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data by skin contact. Mutation data reported. Less acutely toxic than ppropiolactone. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, alcohol foam, CO2, dry chemical. Potentially explosive reaction with butanol + 2,4 dichlorophenol + sodium hydroxide. When heated to decomposition it emits acrid and irritating fumes.
Potential Exposure
Used as a chemical intermediate for making other chemicals, including pesticides, cosmetics, and pharmaceuticals; as a solvent for paint, nail polish removers, and industrial chemicals. Used in electronics, drilling and petroleum industries as a stabilizer and solvent. Used as a flavoring agent in various foods and beverages, including grains and breakfast foods, candy, and alcoholic and nonalcoholic drinks. Drug of abuse: the United States Food and Drug Administration has warned the public not to purchase or consume products, containing gamma-butyrolactone (GBL). FDA has also asked the companies that manufacture these products to voluntarily recall them. The agency has received reports of serious health problems—some that are potentially life-threatening—associated with the use of these products. Although labeled as dietary supplements and marketed under various brand names, these products are illegally marketed unapproved new drugs. False advertising claims include building muscles, improved physical performance, enhanced sex, reduced stress and induced sleep
Shipping
Listed by some sources as unregulated. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Purification Methods
Dry the lactone over anhydrous CaSO4, then fractionally distil it. Handle it in a fume cupboard due to its TOXICITY. [Beilstein 17 V 7.]
Incompatibilities
4-Butyrolactone is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, alcohols, amines, strong and inorganic acids, strong bases. Rapidly hydrolyzed by bases and slowly hydrolyzed by acids. It is hygroscopic and volatile with steam. Combustible; vapor may form explosive mixture with air.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. All federal, state, and local environmental regulations must be observed.
Properties of Gamma Butyrolactone
| Melting point: | -45 °C (lit.) |
| Boiling point: | 204-205 °C (lit.) |
| Density | 1.12 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 1.5 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3291 | 4-HYDROXYBUTANOIC ACID LACTONE |
| Flash point: | 209 °F |
| storage temp. | 2-8°C |
| solubility | DMF: 10 mg/ml; DMSO: 10 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): 1 mg/ml |
| form | neat |
| color | Clear colorless |
| Odor | at 100.00 %. creamy oily fatty caramel |
| PH Range | 4 at 100 g/l at 20 °C |
| explosive limit | 16% |
| Water Solubility | MISCIBLE |
| Merck | 13,1596 |
| JECFA Number | 219 |
| BRN | 105248 |
| Dielectric constant | 40.960000000000001 |
| Stability: | Stable. Hygroscopic. Incompatible with strong oxidizing agents, strong acids, strong bases, strong reducing agents. |
| CAS DataBase Reference | 96-48-0(CAS DataBase Reference) |
| NIST Chemistry Reference | «gamma»-Butyrolactone(96-48-0) |
| IARC | 3 (Vol. 11, Sup 7, 71) 1999 |
| EPA Substance Registry System | .gamma.-Butyrolactone (96-48-0) |
Safety information for Gamma Butyrolactone
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Gamma Butyrolactone
| InChIKey | YEJRWHAVMIAJKC-UHFFFAOYSA-N |
Gamma Butyrolactone manufacturer
ASM Organics
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Y-Butyrolactone extrapure CAS 96-48-0View Details
Y-Butyrolactone extrapure CAS 96-48-0View Details
96-48-0 -
 γ-Butyrolactone CAS 96-48-0View Details
γ-Butyrolactone CAS 96-48-0View Details
96-48-0 -
 Gamma -Butyrolactone CAS 96-48-0View Details
Gamma -Butyrolactone CAS 96-48-0View Details
96-48-0 -
 γ-BUTYROLACTONE For Synthesis CAS 96-48-0View Details
γ-BUTYROLACTONE For Synthesis CAS 96-48-0View Details
96-48-0 -
 γ-Butyrolactone CAS 96-48-0View Details
γ-Butyrolactone CAS 96-48-0View Details
96-48-0 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0