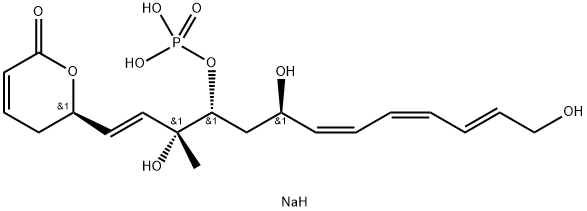
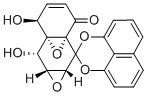
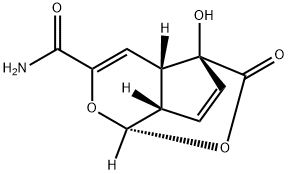
FOSTRIECIN SODIUM SALT
Synonym(s):Fostriecin, Sodium Salt, Streptomyces pulveraceous - CAS 87860-39-7 - Calbiochem;FST, Phosphotrienin
- CAS NO.:87860-39-7
- Empirical Formula: C19H28NaO9P
- Molecular Weight: 454.39
- MDL number: MFCD01939908
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-25 17:59:01
What is FOSTRIECIN SODIUM SALT?
Description
Fostriecin is an inhibitor of the serine/threonine protein phosphatases 2A (PP2A) and 4 (PP4) (IC50s = 3.2 and 3 nM, respectively). It less effectively inhibits topoisomerase II and PP1 (IC50s = 40 and 131 μM, respectively) and does not inhibit PP2B. Through its effects on protein phosphatases, fostriecin increases the level of histone H3 phosphorylation and may alter epigenetic regulation of cell proliferation. On a related note, fostriecin was first identified as an antitumor antibiotic.
The Uses of FOSTRIECIN SODIUM SALT
Antineoplastic. Fostriecin is a Topo II, PP1, and PP2A inhibitor.
The Uses of FOSTRIECIN SODIUM SALT
Fostriecin has been used as a protein phosphatases type 2A (PP2A) inhibitor to study its effects on phosphorylated myosin light chain (pMLC) levels in axon initial segments (AIS). It has also been used as a PP2A inhibitor to study its effects on the regulation of protein kinase Akt (pAKT) by dopamine (DA) in larval zebrafish brain.
What are the applications of Application
Fostriecin is a Topo II, PP1, and PP2A inhibitor
Biological Activity
Originally identified as an antitumor antibiotic from Streptomyces pulveraceous . Displays potent inhibition of protein phosphatase types 2A (PP2A) and 4 (PP4) with IC 50 values of 1.5 nM and 3 nM respectively. Also exhibits weaker inhibition of topoisomerase II (IC 50 = 40 μ M) and protein phosphatase type 1 (PP1) (IC 50 = 131 μ M) with no apparent inhibition of protein phosphatase type 2B (PP2B). Active in vivo .
Biochem/physiol Actions
Fostriecin is a natural inhibitor of serine/ threonine phosphatases. It is a phosphate monoester produced by fermentation beer of Streptomyces pulveraceus. Fostriecin is a strong inhibitor of protein phosphatases type 2A and 4 (PP2A and PP4) and a weak inhibitor of protein phosphatases type 1 and 5 (PP1 and PP5). It exhibits anti-tumor activity against several tumor cells in vitro and L1210 and P388 leukemias in vivo. The anti-tumor activity of fostriecin is due to its ability to interfere with the reversible?phosphorylation?of proteins that are involved in the progression of the cell cycle. It also exhibits cytotoxic effects. Fostriecin can inhibit the DNA, RNA and protein synthesis.
in vitro
a human tumor cloning assay was adopted to investigate the antineoplastic activity of fostriecin. variety of histologic tumor cells exposure to 10 mg/ml fostriecin resulted in 27% in vitro anticancer response. in another study using hela cells, by suppressing the topo ii catalytic activity, 0.22 mm fostriecin could lead to more than 90% inhibition of cancer cell dna synthesis. [2,3]
in vivo
fostriecin showed intensively cytotoxicity against a number of cancer cell lines and had a strong antitumor effect in animals. 10 mg/ml fostriecin could substantially suppress p388 and l1210 leukemias in mice. [2,3]
storage
-20°C (desiccate)
References
[1] zhang x, wu j, fang l, willis wd. the effects of protein phosphatase inhibitors on the duration of central sensitization of rat dorsal horn neurons following injection of capsaicin. mol pain. 2006 jul; 2(23). doi:10.1186/1744-8069-2-23.
[2] scheithauer w, von hoff dd, clark g, shillis jl, elslager ef. in vitro activity of the novel antitumor antibiotic fostriecin (ci-920) in a human tumor cloning assay. eur j cancer res & clin oncol. 1986 aug; 22(8): 921-6.
[3] gedik cm, collins ar. comparison of effects of fostriecin, novobiocin, and camptothecin, inhibitors of dna topoisomerases, on dna replication and repair in human cells. nnucleic acids res. 1990 aug; 18(4):1008-13.
[4] le lh, erlichman c, pillon l, thiessen jj, day a, wainman n, eisenhauer ea, moore mj. phase i and pharmacokinetic study of fostriecin given as an intravenous bolus daily for five consecutive days.invest new drugs. 2004 apr; 22(2):159-67.
Properties of FOSTRIECIN SODIUM SALT
| storage temp. | -20°C |
| solubility | Soluble in H2O |
| form | Colorless solid |
| Water Solubility | Soluble to 100 mM in water |
Safety information for FOSTRIECIN SODIUM SALT
Computed Descriptors for FOSTRIECIN SODIUM SALT
| InChIKey | GMNLFZGMQHSFFV-DSXPUHJBNA-N |
| SMILES | [C@@H](OP(O)(O)=O)(C[C@@H](O)/C=C\C=C/C=C/CO)[C@@](O)(C)/C=C/[C@H]1CC=CC(=O)O1.[NaH] |&1:0,7,17,22,r| |
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran
You may like
-
 Fostriecin sodium salt from Streptomyces pulveraceus CAS 87860-39-7View Details
Fostriecin sodium salt from Streptomyces pulveraceus CAS 87860-39-7View Details
87860-39-7 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9