Fmoc-Tyr(Me)-OH
Synonym(s):Fmoc-Tyr(Me)-OH;N-α-Fmoc-O-methyl-L-tyrosine
- CAS NO.:77128-72-4
- Empirical Formula: C25H23NO5
- Molecular Weight: 417.45
- MDL number: MFCD00153368
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-23 12:43:00
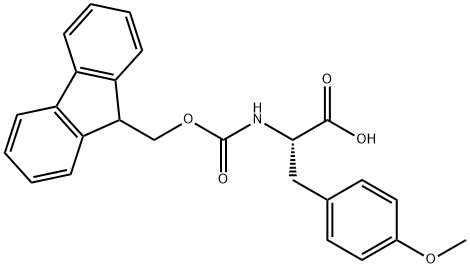
What is Fmoc-Tyr(Me)-OH?
Description
Fmoc-Tyr(Me)-OH, also known as Fmoc-O-methyl-L-tyrosine, is an Fmoc protected tyrosine derivative that is potentially useful for proteomics studies and solid phase peptide synthesis techniques. Tyrosine is a important amino acid - one of the few amino acids which is phosphorylated to vary the physical properties of the peptides, and is a precursor for the formation of iodinated thyroid hormones. The Fmoc group is typically removed with a base such as pyridine - an orthogonal de-protection strategy to the acid labilie Boc group.
Chemical properties
White to off-white powder
What are the applications of Application
Fmoc-O-methyl-L-tyrosine is an Fmoc protected tyrosine derivative
Synthesis
Fmoc-Tyr(Me)-OH, also known as Fmoc-Tyr(OMe)-OH, was obtained from Fmoc-Tyr(OMe)-OMe within 1h and was isolated following protocol 1 or 2 in 96% yield[1]. Under argon atmosphere, anhydrous THF (2.0 mL) was added to the protected amino acid Fmoc-Tyr(OMe)-OMe (0.12 mmol) and to MgI2 * (1.2 mmol). The suspension was heated in a sealed reactor using microwave irradiation at 120°C. A Na2S2O3 aqueous solution (0.1 M) was then added and the resulting homogeneous mixture was directly analysed by analytical HPLC and LC/MS. To isolate the product, two protocols could be applied:
Protocol 1: The crude material was purified by preparative HPLC.
Protocol 2: The crude material was extracted with AcOEt (x3). The organic layer was washed with brine, dried over MgSO4, filtered and concentrated under reduced pressure. The residue was diluted in H2O/EtOH and lyophilized.
References
[1] Berthet M, et al. MgI2 -Mediated Chemoselective Cleavage of Protecting Groups: An Alternative to Conventional Deprotection Methodologies. Chemistry - A European Journal, 2015; 21: 11014-11016.
Properties of Fmoc-Tyr(Me)-OH
| Melting point: | 161 °C |
| Boiling point: | 647.8±55.0 °C(Predicted) |
| Density | 1.274±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| form | powder to crystal |
| pka | 2.97±0.10(Predicted) |
| color | White to Almost white |
| CAS DataBase Reference | 77128-72-4(CAS DataBase Reference) |
Safety information for Fmoc-Tyr(Me)-OH
Computed Descriptors for Fmoc-Tyr(Me)-OH
| InChIKey | JYQODLWFOPCSCS-QHCPKHFHSA-N |
| SMILES | C(O)(=O)[C@H](CC1=CC=C(OC)C=C1)NC(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 N-Fmoc-4-methoxy-L-phenylalanine 98% (HPLC) CAS 77128-72-4View Details
N-Fmoc-4-methoxy-L-phenylalanine 98% (HPLC) CAS 77128-72-4View Details
77128-72-4 -
 Fmoc-Tyr(Me)-OH 96% CAS 77128-72-4View Details
Fmoc-Tyr(Me)-OH 96% CAS 77128-72-4View Details
77128-72-4 -
![N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-O-methyl-L-tyrosine CAS 77128-72-4](https://img.chemicalbook.in//Content/image/CP5.jpg) N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-O-methyl-L-tyrosine CAS 77128-72-4View Details
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-O-methyl-L-tyrosine CAS 77128-72-4View Details
77128-72-4 -
 Fmoc-Tyr(Me)-OH CAS 77128-72-4View Details
Fmoc-Tyr(Me)-OH CAS 77128-72-4View Details
77128-72-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1