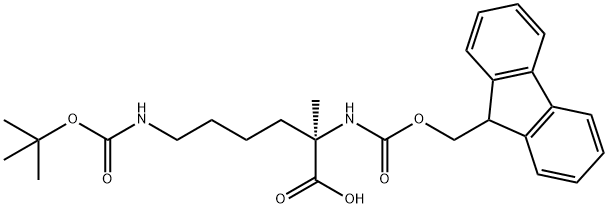
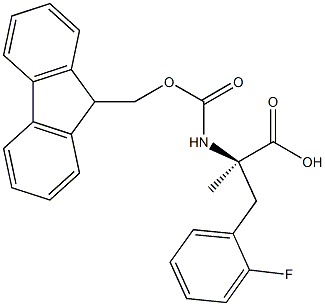
FMoc-α-Me-Lys(Boc)-OH
- CAS NO.:1202003-49-3
- Empirical Formula: C27H34N2O6
- Molecular Weight: 482.57
- MDL number: MFCD17019258
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-27 16:27:43
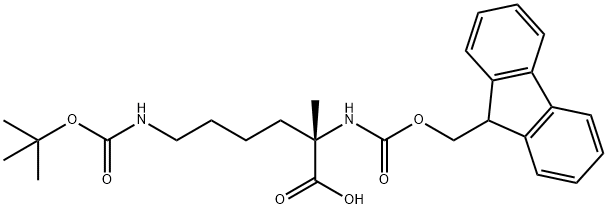
What is FMoc-α-Me-Lys(Boc)-OH?
Chemical properties
White to off-white powder.
The Uses of FMoc-α-Me-Lys(Boc)-OH
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-6-((tert-butoxycarbonyl)amino)-2-methylhexanoic acid is a reagent in the preparation/biological evaluation of a vapreotide analogs containing (S)-α-methyl-lysine.
The Uses of FMoc-α-Me-Lys(Boc)-OH
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-6-((tert-butoxycarbonyl)amino)-2-methylhexanoic acid is a reagent in the preparation/biological evaluation of a vapreotide analogs containing (S)?-?α-?methyl-?lysine.
Synthesis
Benzyl (2R,3S)-(?)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate (4) was successively alkylated with methyl iodide and 1,4-diiodobutane using a base. In each alkylation step anti-alkylated product formed exclusively. The iodo group was displaced with azide, which served as a precursor for the side-chain amino function. Catalytic hydrogenation with concomitant cleavage of the chiral auxiliary afforded (L)-α-Me-Lys-OH (9) in a total of four steps in good yield. (L)-Fmoc-α-Me-Lys(Boc)-OH (16) was obtained from 9 via regioselective benzyloxycarbonylation. Alternately, (L)-Fmoc-α-Me-Lys(Boc)-OH (16) was obtained via Staudinger reduction of azide (8) in a total of six steps in good yield.
Properties of FMoc-α-Me-Lys(Boc)-OH
| Boiling point: | 687.9±55.0 °C(Predicted) |
| Density | 1.196±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| form | powder |
| pka | 3.92±0.41(Predicted) |
| color | white |
Safety information for FMoc-α-Me-Lys(Boc)-OH
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P281:Use personal protective equipment as required. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for FMoc-α-Me-Lys(Boc)-OH
| InChIKey | MYQXEVZHWDILHG-MHZLTWQESA-N |
| SMILES | C(O)(=O)[C@](C)(CCCCNC(OC(C)(C)C)=O)NC(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1