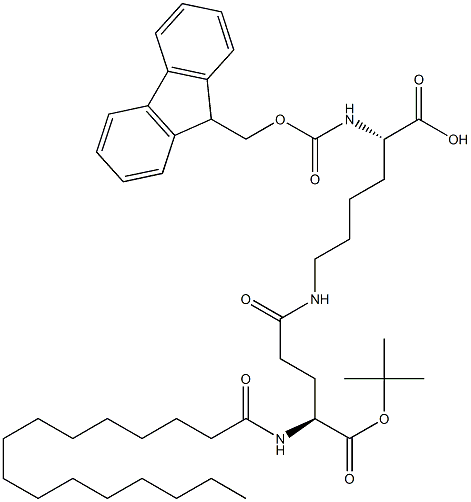
Fmoc-Lys(Pal-Glu-OtBu)-OH
- CAS NO.:1491158-62-3
- Empirical Formula: C46H69N3O8
- Molecular Weight: 792.06
- MDL number: MFCD27952849
- Update Date: 2024-07-26 15:15:06

What is Fmoc-Lys(Pal-Glu-OtBu)-OH?
The Uses of Fmoc-Lys(Pal-Glu-OtBu)-OH
Fmoc-Lys(Pal-Glu-OtBu)-OH can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process.
The Uses of Fmoc-Lys(Pal-Glu-OtBu)-OH
Fmoc-Lys (Pal-Glu-OtBu)-OH is a non-cleavable antibody-drug conjugates linker capable of synthesizing antibody-drug conjugates. It can also be used as an alkyl chain-based PROTAC linker for the synthesis of PROTACs.
What are the applications of Application
Fmoc-Lys(Pal-Glu-OtBu)-OH is a racemic, solid-phase, industrial building block for the synthesis of peptides and polypeptides. This product is used in the synthesis of liraglutide (a peptide), which is used to treat type 2 diabetes mellitus. It is synthesized by a solid phase process using coupling reactions with an acid labile linker. This product has been shown to be an efficient building block for the synthesis of peptides and polypeptides that have a sequence that can be varied by changing the protecting group on the amino acid.
Biological Activity
ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2].
Synthesis

In a flask aqueous sodium carbonate (1.8 g) was prepared followed by addition of THF
(800 mL) and then this mixture was cooled to 0-10 0C. To this mixture, (S)-2-((((9Hfluoren-9-yl)methoxy)carbonyl)amino)-6-aminohexanoic acid (4.59 g) was added.
Then, a solution of 1 -(tert-butyl) 5-(2,5-dioxopyrrolidin-1-yl) palmitoyl-L-glutamate
(corresponding to 5 g of 5-(tert-butoxy)-5-oxo-4-palmitamidopentanoic acid) prepared
according to example 2, was slowly added to above mixture at about 5 0C. The
reaction mixture was stirred for about 2 hours at 25 0C followed by addition of water
(600 mL). The solvents were subjected to distillation and then the pH of the obtained
compound is adjusted to 3.0 using 5N hydrochloric acid. The mixture was stirred for
1-2 hours and resulting solid was isolated filtration, washing with water (30 mL). The
obtained solid was dried under vacuum for 8 hours at about 45°C to afford title
compound in -85% yield.
References
[1] Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337.
[2] Nalawansha DA, et al. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem Biol. 2020;27(8):998-985.
Properties of Fmoc-Lys(Pal-Glu-OtBu)-OH
| Boiling point: | 944.2±65.0 °C(Predicted) |
| Density | 1.099±0.06 g/cm3(Predicted) |
| pka | 3.88±0.21(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Fmoc-Lys(Pal-Glu-OtBu)-OH
Computed Descriptors for Fmoc-Lys(Pal-Glu-OtBu)-OH
| InChIKey | SUFBOZUGILQOQW-FMQAFZDONA-N |
| SMILES | C(O)(=O)[C@@H](N(C1C2=C(C=CC=C2)C2=C1C=CC=C2)C(OC)=O)CCCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCC)C(OC(C)(C)C)=O |&1:3,31,r| |
Fmoc-Lys(Pal-Glu-OtBu)-OH manufacturer
Rushi Pharma
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

![N-[(9H-Fluoren-9-ylMethoxy)carbonyl]-4-[[[(4S)-hexahydro-2,6-dioxo-4-pyriMidinyl]carbonyl]aMino]-L-phenylalanine](https://img.chemicalbook.in/CAS/20150408/GIF/1253282-31-3.gif)
![4-[(AMinocarbonyl)aMino]-N-[(9H-fluoren-9-ylMethoxy)carbonyl]-D-phenylalanine](https://img.chemicalbook.in/CAS/20150408/GIF/324017-22-3.gif)
You may like
-
 Fmoc-l-lys(palm-l-glu-otbu)-oh 95% CAS 1491158-62-3View Details
Fmoc-l-lys(palm-l-glu-otbu)-oh 95% CAS 1491158-62-3View Details
1491158-62-3 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
