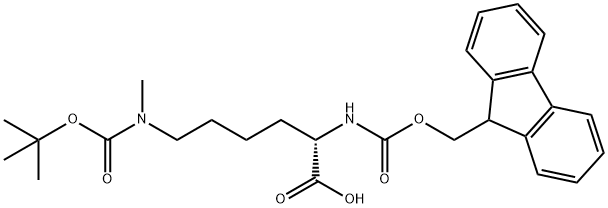
FMOC-LYS(BOC)(ME)-OH
Synonym(s):Fmoc-Lys(Me,Boc)-OH;N-α-Fmoc-N-ε-methyl-N-ε-t.-Boc-L-lysine
- CAS NO.:951695-85-5
- Empirical Formula: C27H34N2O6
- Molecular Weight: 482.57
- MDL number: MFCD01861332
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:06
What is FMOC-LYS(BOC)(ME)-OH?
Description
Fmoc-lys (BOC) (ME)-OH is a kind of protected form of lysine. Lysine is double-protected by butyloxycarbonyl (BOC) and 9H-fluoren-9-ylmethoxycarbon (FMOC). It is a derivative for the introduction of monomethyl-lysine during Fmoc SPPS. Coupling can be carried out using any standard activation method. The Boc protecting group can be removed through the TFA-mediated cleavage reaction. As a kind of protected amino acid, it is an important intermediate in various fields such as peptide synthesis, asymmetric synthesis, medicinal chemistry as well as polymer chemistry.
The Uses of FMOC-LYS(BOC)(ME)-OH
- Heterochromatin protein 1 (HP1) depends on trimethylation of H3 Lys 9; aggregation of chromatines depends on Lys methylation
- Fmoc-Lys(Boc, Me)-OH is useful for preparing monomethylated histone fragments utilized in studying the regulatory roles of methylated histones. Fmoc-Lys(Boc,Me)-OH can be coupled using standard activating procedures. The Boc group on the side epsilon nitrogen atom is removed with TFA, usually at the same time as the peptide is cleaved from the resin.
- Fmoc-Lys(Boc, Me)-OH is a novel derivative for the introduction of monomethyl-lysine during Fmoc SPPS. Coupling can be carried out using any standard activation method. Removal of the Boc protecting group occurs during the course of the TFA-mediated cleavage reaction.
General Description
Fmoc protected N-methylated lysine
References
http://www.chempep.com/ChemPep_Products2_Fmoc-Amino-Acid_Fmoc-Lys_Boc_Me_-OH.htm
https://en.wikipedia.org/wiki/Peptide_synthesis
Tung, C. L., et al. "A fluorogenic probe for recognizing 5-hydroxylysine inspired by serine/threonine ligation. " Chemical Communications 50.40(2014):5298-300.
Properties of FMOC-LYS(BOC)(ME)-OH
| Melting point: | 85-87℃ |
| Boiling point: | 665.4±55.0 °C(Predicted) |
| Density | 1.200 |
| storage temp. | -20°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
| pka | 3.88±0.21(Predicted) |
| color | White to off-white |
Safety information for FMOC-LYS(BOC)(ME)-OH
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for FMOC-LYS(BOC)(ME)-OH
| InChIKey | JHMSFOFHTAYQLS-QHCPKHFHSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 951695-85-5 Fmoc-Lys(Me,Boc)-OH,95% 99%View Details
951695-85-5 Fmoc-Lys(Me,Boc)-OH,95% 99%View Details
951695-85-5 -
 Fmoc-Lys(Me)(Boc)-OH 98% (HPLC) CAS 951695-85-5View Details
Fmoc-Lys(Me)(Boc)-OH 98% (HPLC) CAS 951695-85-5View Details
951695-85-5 -
 Fmoc-Lys(Me,Boc)-OH CAS 951695-85-5View Details
Fmoc-Lys(Me,Boc)-OH CAS 951695-85-5View Details
951695-85-5 -
 Fmoc-Lys(Me,Boc)-OH CASView Details
Fmoc-Lys(Me,Boc)-OH CASView Details -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0