FLUOROSULFONIC ACID
Synonym(s):Fluosulfuric acid
- CAS NO.:7789-21-1
- Empirical Formula: FHO3S
- Molecular Weight: 100.07
- MDL number: MFCD00066174
- EINECS: 232-149-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
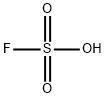
What is FLUOROSULFONIC ACID?
Description
Fluorosulphuric acid (FSO3H) is a free-flowing colourless fuming liquid. It is soluble in polar organic solvents such as acetic acid, ethyl acetate, and nitrobenzene but poorly soluble in nonpolar solvents such as alkanes. FSO3H is corrosive to metals and body tissues, corrodes glass, and is incompatible with many metals and bases. With its strong acidity, FSO3H dissolves almost all organic compounds that are even weak proton acceptors. FSO3H hydrolyses slowly to HF and sulphuric acid (H2SO4). The related triflic acid (CF3SO3H) retains the high acidity of FSO3H but is more hydrolytically stable. FSO3H is one of the strongest known simple Bronsted acids. FSO3H is useful for regenerating mixtures of HF and H2SO4 for etching lead glass. FSO3H isomerises alkanes and the alkylation of hydrocarbons with alkenes, although it is unclear if such applications are of commercial importance. FSO3H is used as a catalyst in organic synthesis and in electroplating and as a fluorinating agent and as a laboratory fluorinating agent. FSO3H has been reported as a highly toxic and corrosive chemical substance. FSO3H is non-combustible but enhances combustion of other substances. It gives off irritating or toxic fumes (or gases) in a fire. FSO3H hydrolyses to release HF. Addition of water to FSO3H causes very violent reactions similar to the addition of water to H2SO4. Addition of FSO3H to water is a much more violent process than addition of H2SO4. The combination of FSO3H and others is categorised as ‘magic acids and super acids’. Very short contact and the fumes of FSO3H cause severe painful burns.
Description
This week’s molecules combine to make “magic”. The first, fluorosulfuric acid (HSO3F; also called fluorosulfonic acid) is an extremely strong Br?nsted acid that has been known since the late 19th century. It is ≈1000 times stronger than pure sulfuric acid, which puts it in the class of superacids. It can protonate and dissolve almost all organic compounds and has several industrial uses, including catalysis and hydrofluorination.
The second molecule, antimony pentafluoride (SbF5), was first reported in 1904. This strong Lewis acid is nasty: It reacts violently with water, attacks the skin and eyes, and corrodes metals. Its chief use is the fluorination of organic compounds.
So where’s the magic? In the 1960s, George A. Olah and co-workers, first at Dow Chemical (Midland, MI), then at Case Western Reserve University (Cleveland), were seeking ways to make and study stable carbocations. To extract hydride groups (hydrogen anions) from organic compounds, they needed a strong, but poorly nucleophilic, acid.
At Case Western, the researchers discovered that a mixture of HSO3F and SbF5easily dissolves paraffins. NMR spectra of the solutions showed that the acidic combination cleaved the long-chain alkanes, and the fragments isomerized to form stable?tert-butyl cations (Me3C+). This result was so spectacular that the chemists dubbed the mixture “magic acid”. The usual molar ratio of the components is 1:1, but other combinations have also been useful.
Olah went on to do extensive research on carbocations at Case Western and then the University of Southern California (Los Angeles). He received the Nobel Prize in Chemistry in 1994 for his pioneering work.
Chemical properties
colourless liquid
The Uses of FLUOROSULFONIC ACID
Catalyst in organic synthesis, electropolishing, fluorinating agent.
Definition
ChEBI: Fluorosulfonic acid is a sulfur oxoacid.
General Description
A fuming liquid. Boiling point 163°C. Density 1.73 g / cm3. Corrosive to metals and to tissue. Both very short contact and the fumes can cause severe painful burns. Used as a catalyst in organic synthesis, in electroplating and as a fluorinating agent.
Air & Water Reactions
Fumes in air. Reacts violently with water to form hydrogen fluoride and sulfuric acid mist.
Reactivity Profile
FLUOROSULFONIC ACID is very strongly acidic [Merck]. Reacts exothermically with chemical bases (examples: amines, amides, and inorganic hydroxides). These reactions can generate dangerously large amounts of heat in small spaces. Reacts violently with water to generate hydrofluoric acid and sulfuric acid. Reacts with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. Generates flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. May catalyze (increase the rate of) chemical reactions.
Hazard
Extremely irritating to eyes and tissue.
Health Hazard
Inhalation of fumes causes severe irritation of nose and throat. Contact of liquid with eyes or skin causes very severe burns of mouth and stomach.
Safety Profile
Probably a poison by inhalation. A corrosive irritant to the skin, eyes, and mucous membranes. See also FLUORIDES, SULFURIC ACID, and FLUOROSULFONATES.
Properties of FLUOROSULFONIC ACID
| Melting point: | −87.3 °C(lit.) |
| Boiling point: | 165.5 °C(lit.) |
| Density | 1.726 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 2.5 mm Hg ( 25 °C) |
| storage temp. | 2-8°C |
| solubility | reacts with H2O |
| pka | -6.29±0.15(Predicted) |
| form | colorless liquid |
| color | colorless |
| Water Solubility | hydrolyzes violently in H2O; reddish?brown color in acetone [MER06] |
| Merck | 13,4206 |
| Stability: | Stable. May corrode glass. Incompatible with many metals, bases. |
| CAS DataBase Reference | 7789-21-1(CAS DataBase Reference) |
| EPA Substance Registry System | Fluorosulfuric acid (7789-21-1) |
Safety information for FLUOROSULFONIC ACID
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for FLUOROSULFONIC ACID
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 7789-21-1 Fluorosulfonic acid 98%View Details
7789-21-1 Fluorosulfonic acid 98%View Details
7789-21-1 -
 Fluorosulfonic acid CAS 7789-21-1View Details
Fluorosulfonic acid CAS 7789-21-1View Details
7789-21-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
