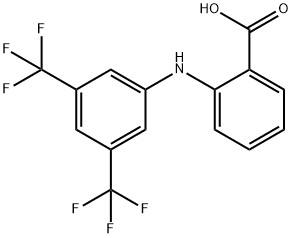
Flufenamic acid
Synonym(s):N-(α,α,α-Trifluoro-m-tolyl)anthranilic acid;N-(3-[Trifluoromethyl]phenyl)anthranilic acid
- CAS NO.:530-78-9
- Empirical Formula: C14H10F3NO2
- Molecular Weight: 281.23
- MDL number: MFCD00002422
- EINECS: 208-494-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
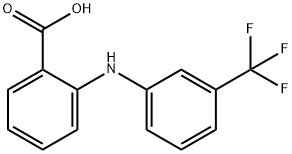
What is Flufenamic acid?
Description
Flufenamic acid (FFA), namely N-(alpha,alpha,alpha-trifluorom-tolyl) anthranilic acid (CI-440), is an aromatic amino acid consisting of anthranilic acid carrying an N-(trifluoromethyl)phenyl substituent. It is an effective drug in the treatment of special types of migraine. Its anti-inflammatory and analgesic effects were recognized in the 1960s (Winder et al., 1963) and thus FFA is included in the family of non-steroidal anti-inflammatory drugs (NSAIDs) with mefenamic, meclofenamic (MFA) and niflumic acids (NA). Anti-inflammatory actions occur mainly through reduction of prostaglandin synthesis from arachidonic acid by inhibiting the cyclo-oxygenases.
Chemical properties
Fluomic acid is a Pale-Yellow or light yellow-green crystal or crystalline powder with bitter taste. It is almost insoluble in water and can be dissolved in 50% ethanol. It is a non-hormonal anti-inflammatory and analgesic drug. It was prepared by photolysis of the corresponding benzotriazinone.
Originator
Flufenamic acid,AroKor Holdings Inc.
The Uses of Flufenamic acid
Flufenamic acid is used for moderate pain and dysmenorrhea, but it should not be used for more than 1 week due to the possibility of nephrotoxicity, gastrointestinal toxicity, and anemia. It is frequently used in combination with the anticoagulant warfarin, the effect of which is strengthened when combined with flufenamic acid.
The Uses of Flufenamic acid
An NSAID found to be a reversible gap junction blocker
What are the applications of Application
Flufenamic acid is a reversible gap junction blocker
Background
An anthranilic acid derivative with analgesic, anti-inflammatory, and antipyretic properties. It is used in musculoskeletal and joint disorders and administered by mouth and topically. (From Martindale, The Extra Pharmacopoeia, 30th ed, p16)
Definition
ChEBI: flufenamic acid is an aromatic amino acid consisting of anthranilic acid carrying an N-(trifluoromethyl)phenyl substituent. An analgesic and anti-inflammatory, it is used in rheumatic disorders. It has a role as an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, a non-steroidal anti-inflammatory drug, a non-narcotic analgesic and an antipyretic. It is an aromatic amino acid and an organofluorine compound. It derives from a diphenylamine, an anthranilic acid and a (trifluoromethyl)benzene. It is a conjugate acid of a flufenamate.
Manufacturing Process
A mixture 31.3 g of o-chlorobenzoic acid, 32.2 g of trifluoromethyl-maminobenzene, 3 g of copper powder, 13.8 g of waterless potassium carbonate and 100 ml amyl alcohol was refluxed for 4 hours. To the cooled mixture was added 25 ml of 10 N solution NaOH and the mixture was concentrated and filtrated. Addition to the filtrate hydrochloric acid and water give a sediment of 2-((3-trifluromethyl)phenyl)aminobenzoic acid. After recrystallization from hexane 2-((3-trifluromethyl)phenyl)aminobenzoic acid have melting point 134-136°C.
brand name
Arlef (Parke-Davis, Sankyo, Japan), Ansatin (Ono, Japan), Felunamin (Hokuriko, Japan), Romafen (Biofarma, Turkey).
Therapeutic Function
Antiinflammatory, Antirheumatic
Biological Activity
Flufenamic acid is a nonsteroidal anti-inflammatory drug (NSAID). Inhibits calcium-activated chloride channels (CaCCs). Also increases currents through TRPC6 channels and inhibits currents through TRPC3 and TRPC7 channels.
Mechanism of action
In addition to COX inhibition flufenamate like other fenamates modifies several ion channel functions, e.g., inhibition of nonselective cation conductance, calcium-activated chloride channels, voltage-gated calcium channels and potassium channels and induces blocking of gap junctions. The relevance of these activities for the analgesic and anti-inflammatory potential of fenamates is unknown.
Clinical Use
Flufenamate is a nonsteroidal anti-inflammatory drug used for the treatment of mild to moderate pain of musculoskeletal, joint or soft-tissue origin. It was marketed in a variety of topical formulations alone or in combination with other ingredients.
Safety
Flufenamic acid is used at 600 – 800 mg/d to provide a beneficial therapeutic effect in chronic polyarthritis. The adverse effects most often encountered are gastrointestinal disturbances.
Synthesis
Flufenamic acid, N-(|á,|á,|á-trifluoro-m-tolyl)anthranylic acid (3.2.18),
is synthesized by the reaction of 2-chlorobenzoic acid with 3-trifluoromethylaniline in the
presence of potassium carbonate and copper filings [78,79].
Metabolism
Not Available
Storage
Store at RT
Properties of Flufenamic acid
| Melting point: | 132-135 °C(lit.) |
| Boiling point: | 373.9±42.0 °C(Predicted) |
| Density | 1.3380 (estimate) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of flufenamic acid in these solvents is approximately 11, 39, and 59 mg/ml, respectively. |
| form | Crystalline Powder or Chunks |
| pka | pKa 3.9 (Uncertain);3.85 (Uncertain) |
| color | Off-white to gray-green |
| Water Solubility | 0.0265 g/L (37 ºC) |
| Merck | 14,4132 |
| BRN | 1996069 |
| CAS DataBase Reference | 530-78-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Flufenamic acid(530-78-9) |
Safety information for Flufenamic acid
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Flufenamic acid
Flufenamic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

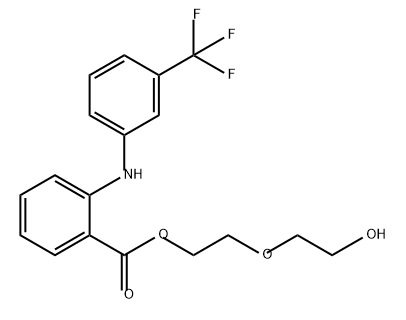
![2-[[3-(Trifluoromethyl)phenyl]amino]benzoic acid oxydi-2,1-ethanediyl ester](https://img.chemicalbook.in/CAS/20150408/GIF/64352-84-7.gif)

You may like
-
 2-(3-Trifluoromethylanilino)benzoic Acid CAS 530-78-9View Details
2-(3-Trifluoromethylanilino)benzoic Acid CAS 530-78-9View Details
530-78-9 -
 Flufenamic acid 98.00% CAS 530-78-9View Details
Flufenamic acid 98.00% CAS 530-78-9View Details
530-78-9 -
 Flufenamic acid CAS 530-78-9View Details
Flufenamic acid CAS 530-78-9View Details
530-78-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
