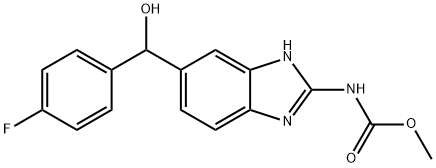
Flubendazole
Synonym(s):Flubendazol;Flubendazole;Flumoxanal;Methyl [5-(4-fluorobenzoyl)-1H-benzimidazol-2-yl]carbamate
- CAS NO.:31430-15-6
- Empirical Formula: C16H12FN3O3
- Molecular Weight: 313.28
- MDL number: MFCD00871999
- EINECS: 250-624-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
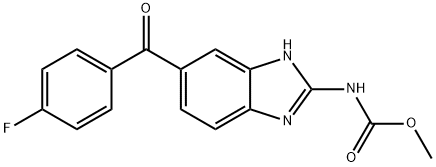
What is Flubendazole?
Description
Flubendazole is a benzimidazole carbamate anthelmintic. It completely eliminates larvae in a mouse model of A. cantonensis infection and exhibits a mean larval reduction of 100% in a T. spiralis infection model when administered at doses of 5 and 50 mg/kg per day, respectively. Flubendazole inhibits mammalian tubulin polymerization (IC50 = 2.5 μM) and inhibits binding of [3H]mebendazole to H. contortus L3 larval tubulin (IC50 = 0.17 μM). Flubendazole also inhibits the proliferation of BT-549, SK-BR-3, MDA-MB-231, and MCF-7 breast cancer cells (IC50s = 0.72, 1.51, 1.75, and 5.51 μM, respectively) and reduces tumor growth in an MDA-MB-231 mouse xenograft model when administered at a dose of 25 mg/kg.
Chemical properties
White Solid
Originator
Fluvermal,Janssen-Le Brun,France,1980
The Uses of Flubendazole
An anthelmintic.
The Uses of Flubendazole
An insectocidal agent.
The Uses of Flubendazole
VETRANAL? analytical standard can be used in the liquid chromatography coupled to mass spectrometry (LC-MS/MS) procedure, performed for the analysis of macrocyclic lactones in milk.
What are the applications of Application
Flubendazole is an insectocidal agent
Definition
ChEBI: A member of the class of mebendazole in which the benzoyl group is replaced by a p-fluorobenzoyl group. A broad-spectrum anthelmintic, it is used, particularly in veterinary medicine, for the treatment of nematodal infections.
Manufacturing Process
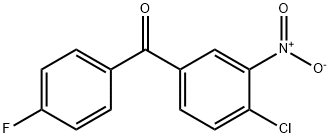
To a stirred and cooled (ice bath) suspension of 25 parts of aluminum chloride
in 52 parts of fluorobenzene is added dropwise a solution of 27.5 parts of 4-
chloro-3-nitrobenzoyl chloride in 52 parts of fluorobenzene. Upon completion,
stirring is continued overnight at room temperature. The reaction mixture is
poured onto water and the product is extracted with methylene chloride. The
extract is washed successively with sodium hydrogen carbonate solution and
water, dried, filtered and evaporated in vacuo. The solid residue is crystallized
from 2-propanol, yielding 4-chloro-4'-fluoro-3-nitrobenzophenone; MP 97.9°C.
A mixture of 24.5 parts of 4-chloro-4'-fluoro-3-nitrobenzophenone, 72 parts of
methanol, 13 parts of sulfolane and 3.12 parts of ammonia is heated in a
sealed tube for 20 hours at 120°C. To the reaction mixture is added
successively 50 parts of water and 25 parts of a diluted hydrochloric acid
solution and the whole is stirred and refluxed for 5 minutes. The reaction
mixture is cooled and the precipitated product is filtered off. It is washed with 2-propanol and recrystallized from 640 parts of toluene, yielding 4-amino-4'-
fluoro-3-nitrobenzophenone; MP 199°C.
A mixture of 14.5 parts of 4-amino-4'-fluoro-3-nitrobenzophenone, 160 parts
of methanol, 6 parts of concentrated hydrochloric acid solution and 0.5 part of
platinum oxide is hydrogenated at normal pressure and at room temperature.
After the calculated amount of hydrogen is taken up, hydrogenation is
stopped. The catalyst is filtered off and the filtrate is evaporated. The residue
is washed with 2-propanol and dried, yielding 3,4-diamino-4'-
fluorobenzophenone hydrochloride; MP 226°C to 230.5°C.
A mixture of 89 parts of S-methylisothiourea sulfate, 6.05 parts of methyl
chloroformate in 7 parts of water is cooled, and at a temperature of 5°C to
10°C, sodium hydroxide solution 25% is added until pH equals 8. Then there
are added successively 6.4 parts of acetic acid, 2.6 parts of sodium acetate
and 8.9 parts of 3,4-diamino-4'-fluorobenzophenone hydrochloride and the
whole is stirred while heating at 85°C for 45 minutes (during this reaction
time, water and 2-propanol is added). The precipitated product is filtered off,
washed with methanol and recrystallized from a mixture of 200 parts of acetic
acid and 80 parts of methanol, yielding methyl N-[5(6)-p-fluorobenzoyl-2-
benzimidazolyl] carbamate; MP > 260°C.
Therapeutic Function
Anthelmintic
General Description
Flubendazol is a broad spectrum anthelmintic, which belongs to the class of compounds known as benzimidazoles. It can be widely used in veterinary and human medicine. It mainly finds applications in deworming poultry, swine, dogs and cats.
Biochem/physiol Actions
Flubendazole, an antithelmintic, has been widely used in treating intestinal parasites. Additionally, Flubendazole has been reported to exert anticancer activities.
Properties of Flubendazole
| Melting point: | 290°C |
| Density | 1.3720 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, in alcohol and in methylene chloride |
| form | neat |
| pka | 10.66±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
| Merck | 14,4118 |
| CAS DataBase Reference | 31430-15-6(CAS DataBase Reference) |
Safety information for Flubendazole
Computed Descriptors for Flubendazole
Flubendazole manufacturer
SETV ASRV LLP
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
 31430-15-6 Flubendazole 99%View Details
31430-15-6 Flubendazole 99%View Details
31430-15-6 -
 FLUBENDAZOLE 31430-15-6 95-99 %View Details
FLUBENDAZOLE 31430-15-6 95-99 %View Details
31430-15-6 -
 Flubendazole 97% CAS 31430-15-6View Details
Flubendazole 97% CAS 31430-15-6View Details
31430-15-6 -
 Flubendazole CAS 31430-15-6View Details
Flubendazole CAS 31430-15-6View Details
31430-15-6 -
 Flubendazol CAS 31430-15-6View Details
Flubendazol CAS 31430-15-6View Details
31430-15-6 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1