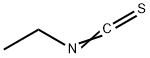
Ethylthiocyanate
Synonym(s):Ethyl rhodanide
- CAS NO.:542-90-5
- Empirical Formula: C3H5NS
- Molecular Weight: 87.14
- MDL number: MFCD00001834
- EINECS: 208-833-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is Ethylthiocyanate?
Chemical properties
clear colorless liquid
Chemical properties
Ethyl thiocyanate is a liquid. Onion odor
The Uses of Ethylthiocyanate
Ethyl thiocyanate was used in the synthesis of hydrogen cyanide by undergoing in situ reduction with dithiothreitol.
General Description
Liquid. Used as an agricultural insecticide.
Reactivity Profile
Nitric acid violently oxidized a thiocyanate solution [Bretherick, 1979 p. 121]. Caution should be exercised in treating a thiocyanate with an oxidizing agent such as a peroxide or chlorate as such mixtures have been known to explode.
Health Hazard
Ethylthiocyanate is highly toxic if ingested.
Fire Hazard
When heated to decomposition, Ethylthiocyanate emits very toxic fumes of oxides of nitrogen and sulfur.
Safety Profile
Poison by ingestion, subcutaneous, intraperitoneal, and intravenous routes. When heated to decomposition it emits very toxic fumes of NO, and SOx. See also THIOCYANATES.
Potential Exposure
This thiocyanate material is used as an agricultural insecticide
Shipping
UN2929 Toxic liquids, flammable, organic, n.o.s., Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Technical Name Required
Purification Methods
Fractionally distil the ester at atmospheric pressure. [Beilstein 2 IV 1218.] (CARE LACHRYMATOR.)
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Esters are generally incompatible with nitrates. Moisture may cause hydrolysis or other forms of decomposition. Caution should be exercised in treating a thiocyanate with an oxidizing agent such as a peroxide or chlorate as such mixtures have been known to explode
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office
Properties of Ethylthiocyanate
| Melting point: | -85.5°C |
| Boiling point: | 145 °C(lit.) |
| Density | 1.012 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 109 °F |
| storage temp. | Flammables area |
| form | liquid |
| color | Colorless to Almost colorless |
| BRN | 1737620 |
| Dielectric constant | 29.6(20℃) |
| CAS DataBase Reference | 542-90-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Thiocyanic acid, ethyl ester(542-90-5) |
| EPA Substance Registry System | Ethyl thiocyanate (542-90-5) |
Safety information for Ethylthiocyanate
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ethylthiocyanate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
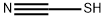


You may like
-
 542-90-5 Ethyl Thiocyanate 99%View Details
542-90-5 Ethyl Thiocyanate 99%View Details
542-90-5 -
 Ethyl Thiocyanate CAS 542-90-5View Details
Ethyl Thiocyanate CAS 542-90-5View Details
542-90-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1