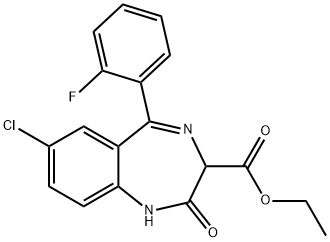
ETHYL LOFLAZEPATE
- CAS NO.:29177-84-2
- Empirical Formula: C18H14ClFN2O3
- Molecular Weight: 360.77
- MDL number: MFCD00868159
- EINECS: 249-489-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
What is ETHYL LOFLAZEPATE?
Originator
Victan,Clin Midy,France,1982
The Uses of ETHYL LOFLAZEPATE
A new benzodiazepine derivative as a new anti-anxiety drug. Ethyl Loflazepate and its metabolite were compared with those of Diazepam (D416855), Nitrazepam (N490140) and Lorazepam (L469850). The anticonflict effect of Ethyl Loflazepate was slightly more potent than that of Diazepam and much more potent than that of Lorazepam. Controlled substance.
Definition
ChEBI: Ethyl loflazepate is an organic molecular entity.
Manufacturing Process
(A) 1-(2-Amino-5-chlorophenyl)-1-(2-fluorophenyl)-2-aza-buty1-en-4-ol: A
mixture of 40 g of 2-methylimidazole hydrochloride and of 90 g of 2-amino-5-
chloro-2'-fluoro-benzophenone in 240 ml of ethanolamine is heated at 135°C
for 2 hours. After cooling, the reaction mixture is poured into an aqueous
sodium bicarbonate solution. The mixture is extracted with ether, the organic
phase is washed repeatedly with water and is dried over sodium sulfate, and
the solvent is evaporated to dryness. The residual oil is chromatographed on a
silica column, elution being carried out with a 50/50 mixture of cyclohexane
and ethyl acetate.
88 g of the expected amine are thus isolated. Melting point: 105°C to 110°C.
(B)1-(2-Amino-5-chlorophenyl)-1-(2-fluorophenyl)-3,3-bis-(ethoxycarbonyl)-2-
aza-prop-1-ene: A mixture of 88 g of the product obtained above, 300 g of
ethyl aminomalonate hydrochloride and 60 ml of acetic acid in 2.3 liters of
absolute ethanol is heated to the reflux temperature for 6 hours. The alcohol
and the acetic acid are evaporated in vacuo and the residue is taken up in
ether. The solution is washed with a dilute sodium bicarbonate solution and
then with water and is dried over sodium sulfate. The solvent is evaporated
and the residue is then chromatographed on a silica column, using a 90/10
mixture of chloroform and ethyl acetate for the elution. An oil (64g) is thus
obtained, and is used, without further treatment, for the cyclization.
A sample recrystallized from isopropyl ether has a melting point of 119°C.
(C) Compound of Code No. CM 6912: 25 g of the imine obtained under (B),
dissolved in 400 ml of acetic acid, are heated at the reflux temperature for 1
hour. After evaporating the solvent in vacuo, the residue is taken up in
methylene chloride. The solution is washed with a dilute sodium bicarbonate
solution and then with water. After evaporating the solvent, the residue is
chromatographed on silica, elution being carried out with an 80/20 mixture of
ether and ethyl acetate. 9 g of benzodiazepine are thus obtained. Melting
point: 196°C.
Therapeutic Function
Tranquilizer
Properties of ETHYL LOFLAZEPATE
| Melting point: | 193-194° |
| Boiling point: | 507.7±50.0 °C(Predicted) |
| Density | 1.3609 (estimate) |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml; Ethanol: 10 mg/ml; Methanol: 1 mg/ml |
| form | A crystalline solid |
| pka | 10.56±0.70(Predicted) |
Safety information for ETHYL LOFLAZEPATE
Computed Descriptors for ETHYL LOFLAZEPATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran


![3-[(4-chlorophenyl)amino]propanoic acid](https://img.chemicalbook.in/CAS/GIF/21617-19-6.gif)
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5