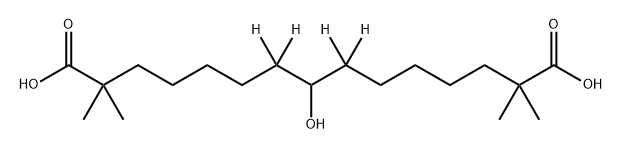
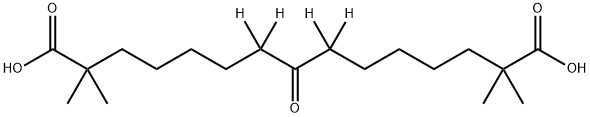
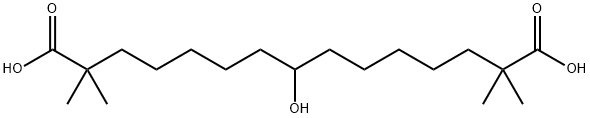
Bempedoic acid
- CAS NO.:738606-46-7
- Empirical Formula: C19H36O5
- Molecular Weight: 344.49
- MDL number: MFCD18800820
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Bempedoic acid?
Absorption
Bempedoic acid is rapidly absorbed in the small intestine. The Tmax of the 180mg tablet is estimated at 3.5 hours.
Toxicity
LD50 information for bempedoic acid is not readily available in the literature. In the case of an overdose with bempedoic acid, contact the local poison control center. To this date, there is no experience with bempedoic acid overdoses. Employ general supportive measures.
Description
Etc-1002 is a small molecule that can be used orally to lower LDL-C and is an activator of LIVER AMPK. ETC-1002 has an effective inhibitory effect on liver ATP-Citrate lyase.
Description
ETC-1002 is a prodrug form of ETC-1002-CoA. ETC-1002 is conjugated to coenzyme A (CoA) by very long-chain acyl-CoA synthetase-1 (ACSVL1) to form ETC-1002-CoA, which inhibits ATP citrate lyase (Ki = 2 μM) and activates AMP-activated protein kinase (AMPK). ETC-1002 suppresses total lipid synthesis in wild-type and AMPKβ1 knockout primary murine hepatocytes. In vivo, ETC-1002 (30 mg/kg) prevents increases in hepatic cholesterol and reduces the size of aortic atherosclerotic lesions induced by a high-fat high-cholesterol diet in Apoe-/-/Ampkβ1-/- (DKO) mice.
The Uses of Bempedoic acid
ETC-1002 (Bempedoic acid, ESP-55016),also known as Bempedoic acid, is an orally available, once-daily LDL-C lowering small molecule designed to lower elevated levels of LDL-C and to avoid side effects associated with existing LDL-C lowering therapies.ETC-1002 is an activator of hepatic AMP-activated protein kinase (AMPK). It has potent inhibitory activity against hepatic ATP-citrate lyase(IC50=29 uM).
Background
High levels of LDL cholesterol (LDL-C) are a major risk factor for cardiovascular events. Caused by genetic mutations or lifestyle factors, hypercholesterolemia can significantly reduce quality of life and increase the risk of mortality from cardiovascular disease. About 1 in 4 patients, or 15 million Americans with elevated LDL-C, are insufficiently managed with maximally tolerated statin therapy alone, requiring additional treatment for hypercholesterolemia.
Bempedoic acid is first-in-class adenosine triphosphate-citrate lyase (ACL) inhibitor used once a day for reducing LDL cholesterol levels in statin-refractory patients. It was developed by Esperion Therapeutics Inc. and approved by the FDA on February 21, 2020. A combination product of bempedoic acid and ezetimibe was approved on February 26, 2020 for increased control of LDL cholesterol levels in patients experiencing refractory elevations despite previous statin treatment.
Indications
Bempedoic acid is indicated as an adjunct to diet and maximally tolerated statin therapy for adults with heterozygous familial hypercholesterolemia or existing atherosclerotic cardiovascular disease that warrants additional lowering of LDL-C.
The combination of bempedoic and ezetimibe is also indicated with diet management and maximally tolerated statin therapy to treat elevated LDL-C levels in adults with heterozygous familial hypercholesterolemia or existing atherosclerotic cardiovascular disease who require further lowering of LDL-C.
Definition
ChEBI: Bempedoic acid is an alpha,omega-dicarboxylic acid that is pentadecanedioic acid which is substituted by methyl groups groups at positions 2 and 14, and by a hydroxy group at position 8. It is a drug used for the treatment of high LDL cholesterol, which is sometimes referred to as 'bad cholesterol'. It has a role as an antilipemic drug, an EC 2.3.3.8 (ATP citrate synthase) inhibitor and a prodrug.
Pharmacokinetics
Bempedoic acid inhibits the synthesis of cholesterol in the liver, reducing LDL-C levels. This reduces the development of atherosclerotic plaques that may increase the risk of cardiovascular events. Earlier clinical trials studying the effects of bempedoic acid showed a dose‐dependent reduction of LDL‐C levels in addition to decreased LDL particle number, and reduced levels of apolipoprotein B, non–HDL cholesterol, and high‐sensitivity C‐reactive protein.
Due to its unique mechanism of action, bempedoic acid is not associated with myositis, an adverse effect that frequently accompanies statin therapy.
More recent trials have supported that this drug significantly decreases LDL-C levels after 12 weeks of therapy and provides additional lowering of LDL-C when combined with ezetimibe and statin therapy. The effects of bempedoic acid on mortality are currently unknown.
Metabolism
The two main metabolites of bempedoic metabolism are ETC-1002-CoA and ESP15228. Bempedoic acid is primarily eliminated via the metabolism of its acyl glucuronide. This drug is reversibly converted to an active metabolite (ESP15228) based on observations during in vitro studies. Both compounds resulting from the metabolism of bempedoic acid are metabolized to become inactive glucuronide conjugates by the enzyme UGT2B7.
Properties of Bempedoic acid
| Melting point: | 87 - 92°C |
| Boiling point: | 506.5±35.0 °C(Predicted) |
| Density | 1.045±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.55±0.45(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C19H36O5/c1-18(2,16(21)22)13-9-5-7-11-15(20)12-8-6-10-14-19(3,4)17(23)24/h15,20H,5-14H2,1-4H3,(H,21,22)(H,23,24) |
Safety information for Bempedoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bempedoic acid
| InChIKey | HYHMLYSLQUKXKP-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C(C)(C)CCCCCC(O)CCCCCC(C)(C)C(O)=O |
Abamectin manufacturer
TAGOOR LABORATORIES PVT LTD
Opulant Pharmaceuticals Pvt Ltd
Basil Drugs AND Pharmaceuticals Pvt Ltd
Ami Lifesciences Pvt Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 738606-46-7 99%View Details
738606-46-7 99%View Details
738606-46-7 -
 Bempedoic Acid 738606-46-7 98%View Details
Bempedoic Acid 738606-46-7 98%View Details
738606-46-7 -
 Bempedoic acid 98%View Details
Bempedoic acid 98%View Details -
 Bempedoic acid 99%View Details
Bempedoic acid 99%View Details -
 Bempedoic acid 97%View Details
Bempedoic acid 97%View Details -
 Bempedoic acid 98%View Details
Bempedoic acid 98%View Details -
 738606-46-7 99%View Details
738606-46-7 99%View Details
738606-46-7 -
 Bempedoic acid 99%View Details
Bempedoic acid 99%View Details
738606-46-7