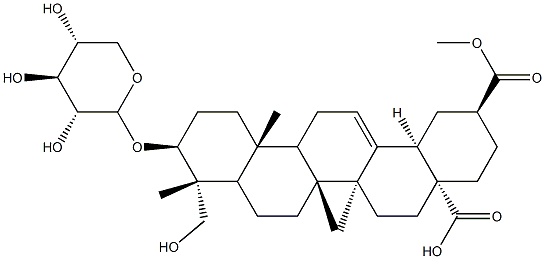
Esculentoside A
- CAS NO.:65497-07-6
- Empirical Formula: C42H66O16
- Molecular Weight: 826.96
- MDL number: MFCD30488995
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
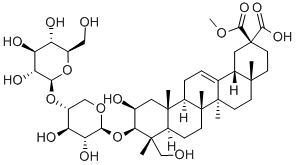
What is Esculentoside A?
The Uses of Esculentoside A
Esculentoside A is a saponin isolated from the root of Phytolacca esculenta. Modulates the immune response. and effects cell proliferation and apoptosis in cells. Anti-inflammatory. 500
Pharmacokinetics
Esculentoside A is a triterpenoid saponin isolated from the root of Phytolacca esculenta. Studies have revealed that this triterpenoid exhibits a strong anti-inflammatory property. Ci et al. (2015) observed the anti-inflammatory potency of esculentoside A against allergic airway inflammation in a mouse model. They found that the drug enhanced nuclear Nrf-2 translocation in the lungs of ovalbumin-challenged mice and also decreased airway inflammation (Ci et al., 2015). Esculentoside A enhanced the intracellular anti-oxidant potentials, reduced Th2 cytokine level and down-regulated the expression of adhesion molecule mRNA in lung tissues. Zhang et al. (2014) found that esculentoside A could rectify carbon tetrachlorideinduced acute liver injury in mice. The drug successfully cured the histopathological damage induced by carbon tetrachloride. Rectification has been also noted through the down-regulation of inflammatory molecules and oxidative stresses associated with PPAR-γ, NF-κB and ERK signal pathways (Zhang et al., 2014). Ma et al. (2013) analyzed the role of esculentoside A on lupus nephritis-induced BXSB mice (where male SB/Le mice and female C57BL/6 mice were hybridized, creating a recombinant inbred species). They observed that esculentoside A application significantly reduced urine protein excretion and improved renal function by promoting apoptosis of glomerular intrinsic cells and renal tubular epithelial cells (Ma et al., 2013). The efficacy was also aided by the down-regulation of inflammatory cytokines.
Wang et al. (2016) elucidated the role of esculentoside A against acetaminophen-induced liver toxicity in mice and observed that Nrf2-regulated the survival mechanism via the AMPK/ Akt/GSK3β pathway.
Properties of Esculentoside A
| Melting point: | >192°C (dec.) |
| Boiling point: | 936.7±65.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.39±0.70(Predicted) |
| color | Pale Yellow |
| Stability: | Hygroscopic |
Safety information for Esculentoside A
Computed Descriptors for Esculentoside A
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
