ERBIUM
Synonym(s):;Erbium
- CAS NO.:7440-52-0
- Empirical Formula: Er
- Molecular Weight: 167.26
- MDL number: MFCD00010987
- EINECS: 231-160-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
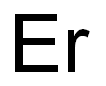
What is ERBIUM?
Chemical properties
grey powder
Physical properties
Erbium is a soft, malleable metal with a silvery metallic luster that only tarnishes (oxidizes)slightly in air. It is one of the rare-earths of the yttrium subgroup of the lanthanide series.
Its melting point is 1,529°C, its boiling point is 2,868°C, and its density is 9.07g/cm3.
Isotopes
There are 39 isotopes of erbium, six of which are stable: Er-162, Er-164, Er-166,Er-167, Er-168, and Er-170. These six isotopes make up the total atomic weight (mass)of erbium, and all the other isotopes are artificially made and short-lived. Their half-livesrange from 200 nanoseconds to 49 hours.
Origin of Name
Named for the quarry in Ytterby, Sweden, where ores and minerals of many elements are found.
Occurrence
Erbium ranks 17th in abundance among the rare-earths, and it is the 46th most abundantelement found in the Earth’s crust. It exists in only 2.5 ppm, meaning that about 2.5 poundsof erbium could be extracted from one million pounds of dirt in the Earth’s crust. Higher concentrationsare found in some areas, but in general, the oxides of erbium are rather scarce.It is found in ores such as monazite, gadolinite, and bastnasite. It was first separated intothree elements in 1843 (yttria, erbia, and terbia). Erbium is also produced as a by-product ofnuclear fission of uranium.
History
Erbium, one of the so-called rare-earth elements of the lanthanide series, is found in the minerals mentioned under dysprosium above. In 1842 Mosander separated “yttria,” found in the mineral gadolinite, into three fractions which he called yttria, erbia, and terbia. The names erbia and terbia became confused in this early period. After 1860, Mosander’s terbia was known as erbia, and after 1877, the earlier known erbia became terbia. The erbia of this period was later shown to consist of five oxides, now known as erbia, scandia, holmia, thulia and ytterbia. By 1905 Urbain and James independently succeeded in isolating fairly pure Er2O3. Klemm and Bommer first produced reasonably pure erbium metal in 1934 by reducing the anhydrous chloride with potassium vapor. The pure metal is soft and malleable and has a bright, silvery, metallic luster. As with other rare-earth metals, its properties depend to a certain extent on the impurities present. The metal is fairly stable in air and does not oxidize as rapidly as some of the other rare-earth metals. Naturally occurring erbium is a mixture of six isotopes, all of which are stable. Twenty-seven radioactive isotopes of erbium are also recognized. Recent production techniques, using ion-exchange reactions, have resulted in much lower prices of the rare-earth metals and their compounds in recent years. The cost of 99.9% erbium metal is about $21/g. Erbium is finding nuclear and metallurgical uses. Added to vanadium, for example, erbium lowers the hardness and improves workability. Most of the rare-earth oxides have sharp absorption bands in the visible, ultraviolet, and near infrared. This property, associated with the electronic structure, gives beautiful pastel colors to many of the rare-earth salts. Erbium oxide gives a pink color and has been used as a colorant in glasses and porcelain enamel glazes.
Characteristics
Although erbium is magnetic at very low temperatures, it is antiferromagnetic and becomesa superconductor at temperatures near absolute zero. It is insoluble in water but soluble inacids. Its salts range from pink to red. Erbium and some of the other rare-earth elements areconsidered to be “impurities” in the minerals in which they are found. Small quantities oferbium can also be separated from several other rare-earths.
The Uses of ERBIUM
Erbium has application in glass coloring, as an amplifier in fiber optics, and in lasers for medical and dental use.
It is commonly used as a photographic filter, and because of its resilience it is useful as a metallurgical additive.
The Erbium ion has a very narrow absorption band coloring erbium salts pink. It is therefore used in eyeware and decorative glassware. It can neutralize discoloring impurities such as ferric ions and produce a neutral gray shade. It is used in a variety of glass products for this purpose.
Lasers based on Er:YAG are ideally suited for surgical applications because of its ability to deliver energy without thermal build-up in tissue.
Erbium Metal, is mainly metallurgical uses. Added to vanadium, for example, Erbium lowers hardness and improves workability. There are also a few applications for nuclear industry. Erbium Metal can be further processed to various shapes of ingots, pieces, wires, foils, slabs, rods, discs and powder.
The Uses of ERBIUM
Erbium has limited commercial use, but it is used as an alloy metal for vanadium to makeit easier to work and to form spring steel. The oxide of erbium is pink, which is used to colorglass and to make lasers that will operate at normal room temperatures. It has limited use ascontrol rods in nuclear fission reactors.
The Uses of ERBIUM
Erbium foil used in Physical Vapor Deposition (PVD) processes, including thermal and electron-beam (e-beam) evaporation, for the preparation of thin films. It is used as an alloying element with titanium and is also used to produce infra-red absorbing glass.
Definition
Element with atomic number 68, aw 167.26, valence of 3; one of the rare-earth elements of the yttrium subgroup.
Definition
A soft malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Erbium has uses in the metallurgical and nuclear industries and in making glass for absorbing infrared radiation. Symbol: Er; m.p. 1529°C; b.p. 2863°C; r.d. 9.066 (25°C); p.n. 68; r.a.m. 167.26.
Definition
erbium: Symbol Er. A soft silverymetallic element belonging to thelanthanoids; a.n. 68; r.a.m. 167.26;r.d. 9.006 (20°C); m.p. 1529°C; b.p.2863°C. It occurs in apatite, gadolinite,and xenotine from certainsources. There are six natural isotopes,which are stable, and twelveartificial isotopes are known. It hasbeen used in alloys for nuclear technologyas it is a neutron absorber; itis being investigated for other potentialuses. It was discovered by CarlMosander (1797–1858) in 1843.
Hazard
Flammable in finely divided form.
Hazard
Erbium nitrate [Er(NO3)3] may explode when “shocked” or at high temperatures. As withother rare-earths, erbium and its compounds should be handled with care because they canbe toxic.
Properties of ERBIUM
| Melting point: | 1529 °C (lit.) |
| Boiling point: | 2868 °C (lit.) |
| Density | 9.062 g/mL at 25 °C (lit.) |
| refractive index | 1.47 (1300 nm) |
| solubility | insoluble in H2O; soluble in acid solutions |
| form | powder |
| color | Silver-gray |
| Specific Gravity | 9.006 |
| Resistivity | 86 μΩ-cm, 20°C |
| Water Solubility | Soluble in acids. Insoluble in water. |
| Sensitive | Moisture Sensitive |
| Merck | 13,3675 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-52-0(CAS DataBase Reference) |
| EPA Substance Registry System | Erbium (7440-52-0) |
Safety information for ERBIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for ERBIUM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




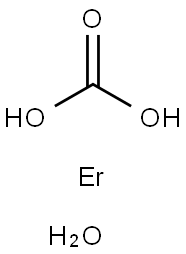
You may like
-
 Erbium, Rare Earth Oxides Content CAS 7440-52-0View Details
Erbium, Rare Earth Oxides Content CAS 7440-52-0View Details
7440-52-0 -
 Erbium, Rare Earth Oxides Content CAS 7440-52-0View Details
Erbium, Rare Earth Oxides Content CAS 7440-52-0View Details
7440-52-0 -
 Erbium CAS 7440-52-0View Details
Erbium CAS 7440-52-0View Details
7440-52-0 -
 Erbium CAS 7440-52-0View Details
Erbium CAS 7440-52-0View Details
7440-52-0 -
 Erbium CAS 7440-52-0View Details
Erbium CAS 7440-52-0View Details
7440-52-0 -
 Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
7440-52-0 -
 Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
7440-52-0 -
 Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
Erbium rod, 12.7mm (0.5 in.) dia. CAS 7440-52-0View Details
7440-52-0