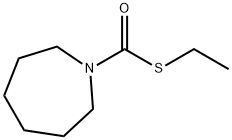
Eradicane
Synonym(s):S-Ethyl-N,N-dipropylthiocarbamate
- CAS NO.:759-94-4
- Empirical Formula: C9H19NOS
- Molecular Weight: 189.32
- MDL number: MFCD00053780
- EINECS: 212-073-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Eradicane?
Chemical properties
Light yellow liquid or yellow granular material. Commercial products may be in the form of dusts, sprays, solutions, wettable powder suspensions or emulsions.
The Uses of Eradicane
Selective systemic herbicide used for preemergence control of perennial and annual grasses such as johnsongrass, nutgrass and quackgrass. Also for control of some broadleaved weeds such as chickweed, henbit, lambsquarters, pigweed and purslane. EPTC is also used in vegetable crops, alfalfa, cotton, flax, pineapple, almonds and walnuts.
The Uses of Eradicane
EPTC is a pesticide, S-Ethyl-N,N-dipropylthiocarbamate.
The Uses of Eradicane
A preemergence herbicide.
Definition
ChEBI: EPTC is a tertiary amine.
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 714, 1959 DOI: 10.1021/ja01512a052
General Description
S-Ethyl dipropylthiocarbamate (EPTC) is a pale to dark yellow liquid with an aromatic odour/pleasant scent characteristic to thiocarbamates. EPTC is a commonly used herbicide. EPTC has a pleasant scent and at 20°C is miscible with most organic solvents, including acetone, ethyl alcohol, kerosene, methyl isobutyl ketone (MIBK), and xylene.
EPTC was the first thiocarbamate herbicide developed. EPTC formulations are used for the control of preemergent weeds, especially grasses. EPTC inhibits photosynthesis, respiration, and the synthesis of lipids, proteins, and RNA in these seedlings. Resistant plants are less sensitive to the herbicidal action apparently due to their ability to rapidly metabolise EPTC.
Air & Water Reactions
Water insoluble. Slowly decomposes in water to form carbon disulfide and propyl amine. Such decompositions are accelerated by acids.
Reactivity Profile
Eradicane may generate flammable gases with aldehydes, nitrides, and hydrides. Incompatible with acids, peroxides, and acid halides.
Health Hazard
SYMPTOMS: Symptoms of exposure to Eradicane may include headache, giddiness, nervousness, blurred vision, weakness, nausea, cramps, diarrhea, sweating, miosis, tearing, salivation, vomiting and cyanosis.
Fire Hazard
Flash point data are not available for Eradicane, but Eradicane is probably combustible.
Flammability and Explosibility
Non flammable
Agricultural Uses
Herbicide: Some formulations are Restricted Group Pesticides (RUP). EPTC is a pre-emergence and early post-emergence herbicide used to control the growth of germinating annual weeds, including broadleaves, grasses, and sedges. It is used in every region of the United States in the production of a wide variety of food crops. The heaviest usage is in the Corn Belt, Northeastern and Mid-Atlantic states, Coastal and Northern Great Plains and in the Pacific Northwest on corn, potatoes, sweet potatoes, dry beans, peas, alfalfa, and snap beans. EPTC is also used on home-grown vegetables and ornamentals. Not approved for use in EU countries. Registered for use in the U.S. and other countries
Trade name
ALIROX®; EPTAM®; EPTAM® 6E; EPTAM 2.3G (granular, 2.3% by weight); EPTAM 10G (granular, 10% by weight); ERADICANE®; GENEP® EPTC; R-1608®; SHORTSTOP®; STAUFFER® R 1608; TORBIN®
Safety Profile
Poison by ingestion, inhalation, and intravenous routes. Moderately toxic by skin contact route. Mutation data reported. An herbicide. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also CARBAMATES
Potential Exposure
EPTC is a pre-emergence and early postemergence thiocarbamate herbicide used to control the growth of germinating annual weeds, including broadleaves, grasses, and sedges. It is used in every region of the United States in the production of a wide variety of food crops. The heaviest usage is in the Corn Belt, Northeastern and Mid-Atlantic states, Coastal and Northern Great Plains and in the Pacific Northwest on corn, potatoes, sweet potatoes, dry beans, peas, alfalfa, and snap beans. EPTC is also used on home-grown vegetables and ornamentals. Some formulations are Restricted Group Pesticides (RUP)
Environmental Fate
Soil. EPTC is rapidly degraded by soil microbes and fungi yielding carbon dioxide,
ethyl mercaptan and amino residues (Kaufman, 1967; Lee, 1984; Hartley and Kidd, 1987).
Degradation occurs via hydrolysis of the ester linkage forming the corresponding mercaptan,
alkylamine (n-dipropylamine) and carbon dioxide. Transthiolation and oxidation of
the mercaptan forms the alcohol which is further oxidized to afford a metabolic pool
(Kaufman, 1967). EPTC partially degraded in both sterile and nonsterile clay soils. Mineralization
was not observed since carbon dioxide was not detected (MacRae and Alex-
ander, 1965). EPTC sulfoxide was also reported as a metabolite identified in soil (Somasundaram
and Coats, 1991) and in corn plants (Casida et al., 1974). The rapid formation
of carbon dioxide was also observed from the microbial degradation of EPTC by a
microbial metabolite isolated from Jimtown loam soil and designated JE1 (Dick et al.,
1990). These researchers proposed that EPTC hydroxylated at the a-propyl carbon forming
the unstable a-hydroxypropyl EPTC which degrades to N-depropyl EPTC and propionaldehyde.
Metabolization of N-depropyl EPTC yields s-ethyl formic acid and propylamine.
Demethylation of s-ethyl formic acid gives s-methyl formic acid. Propylamine and smethyl
formic acid probably degrades to ammonia and methyl mercaptan, respectively
and carbon dioxide (Dick et al., 1990). The reported half-lives in soil ranged from 2 to 4
weeks in two agricultural soils (Regina heavy clay and Weyburn loam) (Smith and Fitzpatrick,
1970) to 30 days (Jury et al., 1987). Rajagopal et al. (1989) reported that the
persistence of EPTC in soil ranged from <4 to 6 weeks when applied at recommended rates.
Plant. EPTC is rapidly metabolized by plants to carbon dioxide and naturally occurring
plants constituents (Humburg et al., 1989).
Chemical/Physical. Emits toxic fumes of phosphorus and sulfur oxides when heated
to decomposition (Sax and Lewis, 1987).
In the gas phase, EPTC reacts with hydroxyl and/or NO3 radicals but not with ozone.
With hydroxy radicals in the presence of NO3, S-ethyl N-formyl-N-propylthiocarbamate
formed as the major product. With NO3 radicals only, the major product was
C3H7(CHO)NC(O)SC2H5. In addition, a minor product formed tentatively identified as
CH3CH2CH2(CH3CH2C(O))NC(O)SCH2CH3. The calculated photolysis lifetimes of EPTC
in the troposphere with hydroxyl and NO3 radicals are 5.8 hours and 5.0 days, respectively
(Kwok et al., 1992).
Metabolic pathway
A new class of xenobiotic metabolite derived from the glutathione conjugate is produced by corn, cotton, and corn cell suspension cultures treated with 14C-EPTC (S-ethyl dipropylthiocarbamate). This metabolite is characterized as S-(N,N-dipropylcarbamoyl)-O- malonyl-3-thiolactic acid and is not detected in soybean plants or peanut cell suspension cultures. A second major metabolite of EPTC, S-(N,N- dipropylcarbamoyl)-N-malonylcysteine, is present in all tissues.
Shipping
UN2902 Pesticides, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Incompatibilities
Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Thermal decomposition of thiocarbamate compounds include carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, andmethylamine. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Land burial is acceptable for small quantities. Larger quantities can be incinerated. (EPTC is combustible and could be incinerated. Recommendable method: Incineration. Peer-review: Incineration in a unit witheffluent gas scrubbing is recommendable for large amount. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Properties of Eradicane
| Melting point: | 25°C |
| Boiling point: | 127°C (20 torr) |
| Density | 0.95 |
| vapor pressure | 3.192Pa at 25℃ |
| refractive index | nD30 1.4750 |
| Flash point: | 116 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| pka | -1.22±0.70(Predicted) |
| color | Clear colorless |
| Water Solubility | 0.375 g/100 mL |
| Merck | 13,3669 |
| BRN | 1762751 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| NIST Chemistry Reference | Carbamothioic acid, dipropyl-, s-ethyl ester(759-94-4) |
| EPA Substance Registry System | S-Ethyl dipropylthiocarbamate (759-94-4) |
Safety information for Eradicane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Eradicane
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 EPTC CAS 759-94-4View Details
EPTC CAS 759-94-4View Details
759-94-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1