Epiandrosterone
Synonym(s):3β-Hydroxy-5α-androstan-17-one;3β-Hydroxyetioallocholan-17-one;5α-Androstan-3β-ol-17-one;Epi-androsterone;Isoandrosterone
- CAS NO.:481-29-8
- Empirical Formula: C19H30O2
- Molecular Weight: 290.45
- MDL number: MFCD00064134
- EINECS: 207-563-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-24 17:44:56
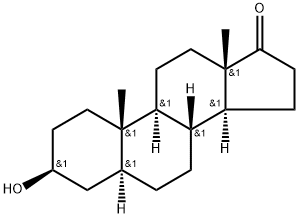
What is Epiandrosterone?
Description
Epiandrosterone is a steroid hormone with weak androgenic activity, derived from 5 α-androstane. It is a dehydroepiandrosterone metabolite and an androgen. It is a precursor of testosterone and estradiol with hypolipidemic and anabolic activities.
Chemical properties
white to off-white crystalline powder. Musky odor when heated.
The Uses of Epiandrosterone
Epiandrosterone is used for weight loss, to improve athletic performance, to reduce sexual problems, and for many other uses, but there is no good scientific evidence to support its use. Epiandrosterone might also be unsafe.
Epiandrosterone is a steroid hormone that is present in normal human urine as a minor constituent , it is a less active 3β-isomer of the androgen Androsterone (A635535). Controlled Substance.
Definition
ChEBI: Epiandrosterone is a 3beta-hydroxy steroid that is (5alpha)-androstane substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It has a role as an androgen and a human metabolite. It is a 17-oxo steroid, a 3beta-hydroxy steroid and an androstanoid. It derives from a 5alpha-androstane.
What are the applications of Application
Epiandrosterone presents some neurological activity since it can bind to the ?-aminobutyric acid (GABA)/benzodiazepine-receptor complex (GABA-RC), acting as a negative non-competitive modulator of GABA-RC, and signaling through the N-methyl-D-aspartate receptor.
It can also inhibit the pentose phosphate pathway, dilating blood vessels pre-contracted by partial depolarization.
Epiandrosterone can inhibit the thromboxane A2 synthesis in activated platelets, decrease plasma plasminogen activator inhibitor type 1, reduce tissue plasminogen activator antigen, improve insulin-like growth factor 1 serum levels, and increase the synthesis of cyclic guanosine monophosphate and nitric oxide.
In vitro assays demonstrate that this compound inhibits Junin virus replication vitro anti-adenovirus (AdV) activity.
Epiandrosterone affects glucose oxidation and interleukin-1 beta in in vitro pancreatic islets.
Preparation
Epiandrosterone is naturally produced by the enzyme 5α-reductase from the adrenal hormone DHEA. Epiandrosterone can also be produced from the natural steroids androstanediol via 17β-hydroxysteroid dehydrogenase or from androstanedione via 3β-hydroxysteroid dehydrogenase.
Side Effects
When taken by mouth: Epiandrosterone is POSSIBLY UNSAFE for most people when taken by mouth. Side effects include infertility, behavioral changes, hair loss, and breast development (in men). Epiandrosterone can also lead to liver damage and heart disease.
in vitro
it was reported that epi, at concentrations from 10 to 100 mm, decreased left-ventricular developed pressure (lvdp) and myocardial contraction rate dose-dependently. in addition, epi also increased cpp in isolated hearts, down-regulated levels of myocardial nadph and nitrite, as well as relaxed rat aortic rings in the dose-dependent manner. findings from whole cell clamp via electrophysiological analysis of single ventricular myocytes demonstrated that epi could reversibly block l-type channel currents carried by ba2+ in a dose-dependent manner with an ic50 of2 ± 6 m. moreover, epi, at a concentration of 30 mm, accelerated the decay of iba during depolarization, which suggested this agent as a l-type ca2+ channel antagonist with similar properties to those of 1, 4-dihydropyridine (dhp) ca2+ channel blockers. [1]
in vivo
in vivo tests were performed using g-6-pd-low c57l/j mouse erythrocytes. every other day, mice were orally administered with 450 or 900 mg/kg of tested agents including dhea, epi, pregnenolone (preg) and androstanedione (andr) for seven days (four doses). three hours after the final dose, mice were sacrificed. findings from blood samples suggested that g-6-pd activity had no significant changes, which might be caused by the lack of receptor sites for the steroids on the erythrocyte membrane. [2]
Purification Methods
Purify epi-androsterone via the acetate, hydrolyse this and recrystallise it from CHCl3/hexane or aqueous EtOH. The acetate [1239-31-2] is purified by chromatography and when crystallised from pet ether has m 103-104o, []D +68.5o (c 1, CHCl3). The oxime has m 194-196o (from MeOH), []D +17.5o (c 6.2, CHCl3). The racemic ketone is sublimed at 130o/high vacuum and after two crystallisations from methylcyclohexane it gives prisms with m 161-162o (which changed crystal form at 140-145o). [Ruzicka & Wettstein Helv Chim Acta 18 1264 1935, Johnson et al. J Am Chem Soc 75 2275 1953, 78 6331 1956, Cordwell et al. J Chem. Soc 361 1953, Beilstein 8 IV 462.]
Mode of action
Epiandrosterone is a dehydroepiandrosterone metabolite and a precursor of testosterone and estradiol with hypolipidemic and anabolic property. Epiandrosterone, a potential neurosteroid, appears to bind to the gamma-aminobutyric acid (GABA)/benzodiazepine-receptor complex (GABA-RC), acting as a negative non-competitive modulator of GABA-RC as well as signal through the N-methyl-D-aspartate receptor. In addition this agent inhibits the pentose phosphate pathway (PPP) thereby dilating blood vessels pre-contracted by partial depolarization. Also, epiandrosterone inhibits the synthesis of thromboxane A2 in activated platelets, reduces plasma plasminogen activator inhibitor type 1 and tissue plasminogen activator antigen, increases serum levels of insulin-like growth factor 1 and increases cyclic guanosine monophosphate and nitric oxide synthesis. These effects may improve circulation in the microvasculature.
References
[1]gupte sa, tateyama m, okada t, oka m and ochi r. epiandrosterone, a metabolite of testosterone precursor, blocks l-type calcium channels of ventricular myocytes and inhibits myocardial contractility. j mol cell cardiol. 2002 mar; 34: 679- 88.
[2]calabrese ej, horton hm and leonard da. the in vivo effects of four steroids on glucose-6-phosphate dehydrogenase activity of c57l/j mouse erythrocytes. j. environ. sci. health. 1987; a22(6): 563-74.
[3]forrest ad, drewery j, fotherby k and laverty sg. a
Properties of Epiandrosterone
| Melting point: | 172-174 °C |
| Boiling point: | 372.52°C (rough estimate) |
| alpha | 91 º (c=1, CH3OH) |
| Density | 1.0320 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| storage temp. | Refrigerator |
| solubility | insoluble in H2O; ≥12.35 mg/mL in EtOH; ≥28.27 mg/mL in DMSO |
| form | solid |
| pka | 15.14±0.60(Predicted) |
| color | White to off-white |
| Water Solubility | practically insoluble |
| Merck | 3609 |
| BRN | 1884007 |
| CAS DataBase Reference | 481-29-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 3Beta-hydroxy-5alpha-androstan-17-one(481-29-8) |
Safety information for Epiandrosterone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Epiandrosterone
| InChIKey | QGXBDMJGAMFCBF-LUJOEAJASA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![Androstan-17-one,3-[[(4-methylphenyl)sulfonyl]oxy]-,(3,5)-](https://img.chemicalbook.in/CAS/20180808/GIF/10429-07-9.gif)
You may like
-
 trans-Androsterone 99% (GC) CAS 481-29-8View Details
trans-Androsterone 99% (GC) CAS 481-29-8View Details
481-29-8 -
 trans-Androsterone CAS 481-29-8View Details
trans-Androsterone CAS 481-29-8View Details
481-29-8 -
 3BETA-HYDROXY-5ALPHA-ANDROSTAN-17-ONE CAS 481-29-8View Details
3BETA-HYDROXY-5ALPHA-ANDROSTAN-17-ONE CAS 481-29-8View Details
481-29-8 -
 Epiandrosterone USPView Details
Epiandrosterone USPView Details
481-29-8 -
 EpiandrosteroneView Details
EpiandrosteroneView Details
481-29-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
