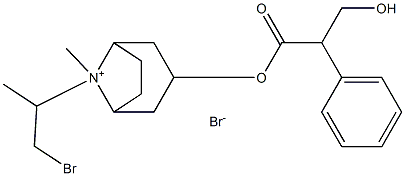
endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate
- CAS NO.:22235-81-0
- Empirical Formula: C19H27NO3
- Molecular Weight: 317.42
- MDL number: MFCD28898375
- EINECS: 2448591
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
![endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate Structural](https://img.chemicalbook.in/CAS/GIF/22235-81-0.gif)
What is endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate?
Chemical properties
Off-White to Pale Orange Solid
The Uses of endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate
N-Isopropyl Noratropine is a Noratropine derivative, an intermediate in the production of Ipratropium.
Biological Activity
n-isopropylnoratropine is a noratropine derivative and an intermediate in the production of ipratropium, an atropine-like bronchodilator drug via an anticholinergic pathway. n-isopropylnoratropine is used to examine the stability of ipratropium [1].muscarinic acetylcholine receptors are acetylcholine receptors that form g protein-receptor complexes in the cell membranes of certain neurons and other cells.n-isopropylnoratropine is an intermediate in the production of ipratropium and used to examine the stability of ipratropium. ipratropium exhibits broncholytic action by reducing the influence of cholinergic on the bronchial musculature. ipratropium blocks muscarinic acetylcholine receptors and therefore promotes the degradation of cgmp. ipratropium bromide (ib) is an anticholinergic bronchodilatory agent to treat chronic obstructive pulmonary disease. in anesthetized dogs, ipratropium with 0.01 and 0.1 mg/ml constricted the airways to 22 +/- 2% and 20 +/- 3% of control, respectively, whereas larger concentrations caused bronchodilation [2]. in moderate asthmatic patients, ipratropium bromide (ib) induced a significant shift of dose-response curve to inhalation of substance p (sp), suggesting that bronchoconstriction induced by sp could be attributed to a weak cholinergic activation [3].
References
[1]. kasawar gb, farooqui m. development and validation of a stability indicating rp-hplc method for the simultaneous determination of related substances of albuterol sulfate and ipratropium bromide in nasal solution. j pharm biomed anal. 2010 may 1;52(1):19-29.
[2]. groeben h, brown rh. ipratropium decreases airway size in dogs by preferential m2 muscarinic receptor blockade in vivo. anesthesiology. 1996 oct;85(4):867-73.
[3]. crimi n, palermo f, oliveri r, et al. influence of antihistamine (astemizole) and anticholinergic drugs (ipratropium bromide) on bronchoconstriction induced by substance p. ann allergy. 1990 aug;65(2):115-20.
Properties of endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate
| Melting point: | 112-114°C |
| Boiling point: | 448.8±45.0 °C(Predicted) |
| Density | 1.15±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.12±0.10(Predicted) |
| color | White to Pale Orange |
Safety information for endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate
Computed Descriptors for endo-(±)-8-aza-8-isopropylbicyclo[3.2.1]oct-3-yl (hydroxymethyl)phenylacetate
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
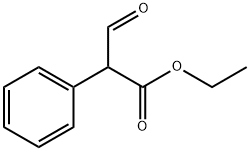
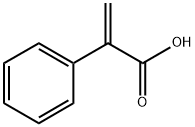
![8-Azabicyclo[3.2.1]octan-3-ol,8-(1-methylethyl)-(9CI)](https://img.chemicalbook.in/CAS/20200515/GIF/259092-15-4.gif)
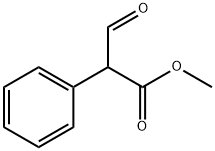
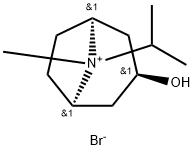
![endo-8-isopropyl-8-azabicyclo[3.2.1]octan-3-ol](https://img.chemicalbook.in/CAS/GIF/3423-25-4.gif)
You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
