ELLIPTICINE
Synonym(s):5,11-Dimethyl-6H-pyrido[4,3-b]carbazole;Ellipticine - CAS 519-23-3 - Calbiochem
- CAS NO.:519-23-3
- Empirical Formula: C17H14N2
- Molecular Weight: 246.31
- MDL number: MFCD00010524
- EINECS: 208-264-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
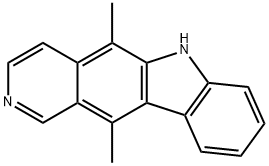
What is ELLIPTICINE?
Description
Ellipticine is an alkaloid isolated from Apocyanaceae plants that exhibits antitumor activities by intercalating into DNA and/or inhibiting DNA topoisomerase II. It forms covalent adducts in DNA after being enzymatically activated with cytochrome P450 isoforms (e.g., CYP3A4, CYP1A1, or CYP1A2) or by peroxidases in target tissues. Ellipticine has been shown to inhibit the proliferation of human breast adenocarcinoma MCF-7 cells, leukemia HL-60 and CCRF-CEM cells, neuroblastoma IMR-32, UKF-NB-3, and UKF-NB-4 cells, and U87MG glioblastoma cells with IC50 values ranging from 0.27-4.7 μM.
The Uses of ELLIPTICINE
Ellipticine is a DNA intercalating agent and exhibits antitumor activities.
What are the applications of Application
Ellipticine is a DNA intercalating inhibitor of Topo II
Definition
ChEBI: A organic heterotetracyclic compound that is pyrido[4,3-b]carbazole carrying two methyl substituents at positions 5 and 11.
Synthesis Reference(s)
Journal of the American Chemical Society, 102, p. 1457, 1980 DOI: 10.1021/ja00524a059
The Journal of Organic Chemistry, 72, p. 7106, 2007 DOI: 10.1021/jo070774z
Tetrahedron Letters, 18, p. 4663, 1977 DOI: 10.1016/S0040-4039(01)83595-4
General Description
A topoisomerase II inhibitor. Acts as an intercalative alkaloid that stimulates topoisomerase II-mediated DNA breakage. Is also capable of uncoupling mitochondrial oxidative phosphorylation.
Biochem/physiol Actions
Cell permeable: yes
in vitro
treatment mammalian dna topoisomerase ii reaction mixture with ellipticine resulted in the stimulation of dna cleavage. the drug-stimulation of dna cleavage is related to the formation of a ternary complex between topoisomerase ii, dna, and ellipticine. ellipticine dose not inhibit enzyme-mediated dna religation, however, it stimulates dna breakage by enhancing the forward rate of cleavage [2]. ellipticine showed growth inhibition activity on l1210 lymphocytic leukemia cells with a ic50 of 0.99 μm [1].
in vivo
ellipticine was evaluated in p. berghei infected mice in the 4-day suppressive test. ellipticine had a 100% inhibition versus controls on days 5 and 7 at an oral dose of 50 mg/kg/day, and the mean survival time (mst) was more than 40 days [3].
Purification Methods
This DNA intercalator is purified by recrystallisation from CHCl3 or MeOH and is dried in vacuo. The UV max values in aqueous EtOH/HCl are at 241, 249, 307, 335 and 426nm. [Marini-Bettolo & Schmutz Helv Chim Acta 42 2146 1959.] The methiodide has m 360o(dec), with UV (EtOH/KOH) at 223, 242, 251, 311, 362 and 432nm. [Goodwin et al. J Am max Chem Soc 81 1903 1959, Beilstein 23/9 V 417.]
References
[1] paoletti c, cros s, xuong nd, lecointe p, moisand a. comparative cytotoxic and antitumoral effects of ellipticine derivatives on mouse l 1210 leukemia. chem biol interact. 1979 apr;25(1):45-58.
[2] tewey km, chen gl, nelson em, liu lf. intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian dna topoisomerase ii. j biol chem. 1984 jul 25;259(14):9182-7.
[3] rocha e silva lf, montoia a, amorim rc, melo mr, henrique mc, nunomura sm, costa mr, andrade neto vf, costa ds, dantas g, lavrado j, moreira r, paulo a, pinto ac, tadei wp, zacardi rs, eberlin mn, pohlit am. comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. phytomedicine. 2012 dec 15;20(1):71-6.
Properties of ELLIPTICINE
| Melting point: | 316-318°C |
| Boiling point: | 379.31°C (rough estimate) |
| Density | 1.257±0.06 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5794 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO: soluble |
| form | Bright yellow solid |
| pka | 16.59±0.40(Predicted) |
| color | Light yellow to yellow |
| Merck | 13,3581 |
| CAS DataBase Reference | 519-23-3(CAS DataBase Reference) |
Safety information for ELLIPTICINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for ELLIPTICINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
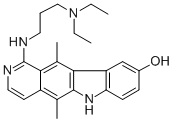
![N,N-diethyl-N'-(9-methoxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazol-1-yl)propane-1,3-diamine](https://img.chemicalbook.in/CAS/GIF/72238-02-9.gif)
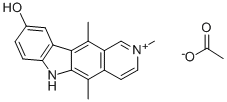
![9-hydroxy-2,5,11-trimethyl-6H-pyrido[4,3-b]carbazolium iodide](https://img.chemicalbook.in/CAS/GIF/58447-24-8.gif)
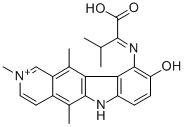
![9-methoxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole](https://img.chemicalbook.in/CAS/GIF/10371-86-5.gif)
You may like
-
 Ellipticine, ≥99% CAS 519-23-3View Details
Ellipticine, ≥99% CAS 519-23-3View Details
519-23-3 -
 Ellipticine CAS 519-23-3View Details
Ellipticine CAS 519-23-3View Details
519-23-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1