Trabectedin
- CAS NO.:114899-77-3
- Empirical Formula: C39H43N3O11S
- Molecular Weight: 761.84
- MDL number: MFCD00874849
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-08 15:36:51
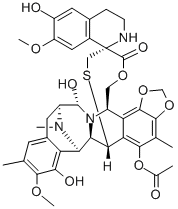
What is Trabectedin?
Absorption
Administered intravenously.
Description
Trabectedin is a marine natural product derived from the tunicate Ecteinascidia turbinate. With its demonstrated in vitro and in vivo activity against a range of solid tumor cell lines, human xenografts and tumor explants, this antineoplastic agent has been developed and launched for the treatment of advanced STS after failure of first-line therapy with anthracyclines or ifosfamide or in patients who are unsuited to receive these agents. Its proposed mechanism of action involves binding to the N2 position of guanine in the minor groove demonstrating a preference for sequences containing 5′-PuGC and 5′-PyGG motifs. Subsequent alkylation of DNA, via an iminium intermediate generated from an intra-molecular acid-catalyzed activation and dehydration of the carbinolamine, induces a curvature of the DNA toward the major groove that ultimately disrupts the binding of transcription factors involved in cell proliferation. Evaluation of trabectedin against the National Cancer Institute’s human in vitro cell line panel, including melanoma, non-small-cell lung, ovarian, renal, prostate, and breast cancer, demonstrated potencies ranging from 1 pM to 10 nM.
Originator
University of Illinois (US)
The Uses of Trabectedin
A tetrahydroisoquinoline alkaloid with antitumor activity isolated from the Caribbean tunicate, Ecteinascidin turbinata. It is the first marine anticancer agent approved in the European Union for patients with soft tissue sarcoma (STS).
The Uses of Trabectedin
A tetrahydroisoquinoline alkaloid with antitumor activity isolated from the Caribbean tunicate, Ecteinascidin turbinata. It is the first marine anticancer agent approved in the European Union for patients with soft tissue sarcoma (STS). Antineoplastic.
Background
Trabectedin, also referred as ET-743 during its development, is a marine-derived antitumor agent discovered in the Carribean tunicate Ecteinascidia turbinata and now produced synthetically. Trabectedin has a unique mechanism of action. It binds to the minor groove of DNA interfering with cell division and genetic transcription processes and DNA repair machinery. It is approved for use in Europe, Russia and South Korea for the treatment of advanced soft tissue sarcoma. It is currently under evaluation for the treatment of breast cancer, prostate cancer, in addition to pediatric sarcomas. Both the European Commission and the U.S. Food and Drug Administration (FDA) have approved trabectedin as an orphan drug in soft tissue sarcomas and ovarian cancer. On October 23, 2015, the FDA approved trabectedin, (as Yondelis), for the treatment of specific soft tissue sarcomas.
Indications
Indicated for treatment of advanced soft tissue sarcoma in patients refractory to or unsuitable to receive anthracycline or ifosfamide chemotherapy in Europe, Russia and South Korea. Approved for orphan drug status by the U.S. FDA for treatment of soft tissue sarcomas and ovarian cancer. Investigated for use/treatment in cancer/tumors (unspecified), gastric cancer, ovarian cancer, pediatric indications, sarcoma, and solid tumors.
Definition
ChEBI: A tetrahydroisoquinoline alkaloid obtained from a Caribbean tunicate Ecteinascidia turbinata. Used for the treatment of soft tissue sarcoma and relapsed ovarian cancer.
brand name
Yondelis
Pharmacokinetics
Two of the rings in the drug's structure allows it to covalently bind to the minor groove of DNA. The third ring protrudes from the DNA which lets it interact with nearby nuclear proteins. This has the additive effect of blocking cell division at the G2 phase.
Clinical Use
Antineoplastic agent:
Advanced soft tissue sarcoma
Ovarian cancer
Side Effects
The most common adverse events included nausea, fatigue, vomiting, anorexia, neutropenia, and increases in aspartate aminotransferase and alanine aminotransferase liver enzymes. To combat the nausea and vomiting, all patients must receive 20mg of dexamethasone intravenously before the trabectedin infusion. Not only does this pretreatment have an antiemetic effect, it also appears to offer a hepatoprotective benefit. Concomitant administration of potent inhibitors and inducers of CYP3A4 should be avoided since plasma levels of this CYP3A4- metabolized drug will be affected. Trabectedin is contraindicated in patients with a known hypersensitivity to trabectedin, concurrent serious or uncontrolled infection, or breast-feeding. Trabectedin should also not be administered in combination with yellow fever vaccine. Finally, there are strict criteria regarding absolute neutrophil count, platelet count, and renal and hepatic enzyme levels for permission to initiate or continue treatment with trabectedin.
Synthesis
While the challenging total synthesis of trabectedin has been accomplished by a few research groups, the commercial preparation begins from readily available cyanosafracin B in 21 steps. Following the Boc and MOM protection of the amine and phenol functionalities, respectively, the methoxy-pquinone was hydrolyzed with sodium hydroxide in methanol. The resulting quinine was reduced (hydrogen over Pd/C) to give an unstable hydroquinone that was subsequently alkylated with bromochloromethane and allyl bromide. The MOM and Boc groups were then removed followed by cleavage of the amide by Edman degradation (through formation of the thiourea with phenyl isothiocyanate and treatment with HCl in 1,4-dioxane). At this point, the amine was protected as the TROC carbamate before reprotecting the phenol as the MOM ether and then liberating the amine with zinc in acetic acid. The amine was converted to an alcohol moiety with sodium nitrite in acetic acid, and this handle was acylated with (S)-N-[(trichloroethoxy)carbonyl]-S-(9-fluorenylmethyl)cysteine. Removal of the allyl-protecting group followed by oxidation provided an 492 Shridhar Hegde and Michelle Schmidt alpha-hydroxy ketone intermediate. Dehydration and deprotection of the cysteine established the Michael addition of the thiol to the o-quinone methide with concomitant trapping with acetic anhydride. The remaining steps involved removal of protecting groups, installation of the tetrahydroisoquinoline ring via a Pictet Spengler reaction, and conversion of the cyano to the alcohol of the carbinolamine with silver nitrate in acetonitrile and water.
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: Avoid concomitant use.
Antibacterials: concentration reduced by rifampicin.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Vaccines: risk of generalised infections - avoid.
Metabolism
Metabolism
Metabolised in the liver, mainly by cytochrome P450 isoenzyme CYP3A4. Excreted mainly via the faeces.
Properties of Trabectedin
| Melting point: | >143°C (dec.) |
| alpha | D25 +114° (c = 0.1 in methanol) |
| Density | 1.55±0.1 g/cm3 (20 ºC 760 Torr) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.73±0.40(Predicted) |
| color | Light Yellow to Yellow |
Safety information for Trabectedin
Computed Descriptors for Trabectedin
| InChIKey | PKVRCIRHQMSYJX-HAHYLZASNA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran
![Spiro[6,16-(epithiopropanoxymethano)-7,13-imino-12H-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1'(2'H)-isoquinolin]-19-one, 3',4',6,6a,7,13,14,16-octahydro-5,6',8,14-tetrahydroxy-7',9-dimethoxy-4,10,23-trimethyl-, (1'R,6R,6aR,7R,13S,14S,16R)- (9CI)](https://img.chemicalbook.in/CAS/20210111/GIF/493024-42-3.gif)
![Spiro[6,16-(epithiopropanoxymethano)-7,13-imino-12H-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1'(2'H)-isoquinolin]-19-one, 5-(acetyloxy)-3',4',6,6a,7,13,14,16-octahydro-6',8-dihydroxy-7',9-dimethoxy-4,10,23-trimethyl-, (1'R,6R,6aR,7R,13S,16R)- (9CI)](https://img.chemicalbook.in/CAS/20211123/GIF/114899-28-4.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8