DYSPROSIUM
Synonym(s):;Dysprosium
- CAS NO.:7429-91-6
- Empirical Formula: Dy
- Molecular Weight: 162.5
- MDL number: MFCD00010982
- EINECS: 231-073-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is DYSPROSIUM?
Chemical properties
metal ingots
Physical properties
Dysprosium is a dense (specific gravity = 8.540) metal. It is soft, and when cut with aknife, it appears as a silvery metal that oxidizes slowly at room temperatures. The white oxide(Dy2O3) that forms on the outside of the metal sloughs off, exposing a fresh surface of themetal for more oxidation. The oxide of dysprosium is also called dysprosia.
Its melting point is 1,412°C, its boiling point is 2,567°C, and its density is 8.540g/cm3.
Isotopes
There are a total of 39 isotopes of dysprosium, seven of which are stable. Theatomic mass of the stable isotopes ranges from 156 to 164 amu (atomic mass units oratomic weight). The unstable isotopes of dysprosium have half-lives ranging from 150milliseconds to 3.0×10+6 years. All of the unstable isotopes are radioactive and are producedartificially.
Origin of Name
The word dysprosium was derived from the Greek word dysprositos, which means “difficult to approach.”
Occurrence
Dysprosium is the 43rd most abundant element on Earth and ranks ninth in abundanceof the rare-earths found in the Earth’s crust. It is a metallic element that is usually found asan oxide (disprosia). Like most rare-earths, it is found in the minerals monazite and allanite,which are extracted from river sands of India, Africa, South America, and Australia and thebeaches of Florida. It is also found in the mineral bastnasite in California.
History
Dysprosium was discovered in 1886 by Lecoq de Boisbaudran, but not isolated. Neither the oxide nor the metal was available in relatively pure form until the development of ion-exchange separation and metallographic reduction techniques by Spedding and associates about 1950. Dysprosium occurs along with other so-called rare-earth or lanthanide elements in a variety of minerals such as xenotime, fergusonite, gadolinite, euxenite, polycrase, and blomstrandine. The most important sources, however, are from monazite and bastnasite. Dysprosium can be prepared by reduction of the trifluoride with calcium. The element has a metallic, bright silver luster. It is relatively stable in air at room temperature, and is readily attacked and dissolved, with the evolution of hydrogen, by dilute and concentrated mineral acids. The metal is soft enough to be cut with a knife and can be machined without sparking if overheating is avoided. Small amounts of impurities can greatly affect its physical properties. While dysprosium has not yet found many applications, its thermal neutron absorption cross-section and high melting point suggest metallurgical uses in nuclear control applications and for alloying with special stainless steels. A dysprosium oxide-nickel cermet has found use in cooling nuclear reactor rods. This cermet absorbs neutrons readily without swelling or contracting under prolonged neutron bombardment. In combination with vanadium and other rare earths, dysprosium has been used in making laser materials. Dysprosium-cadmium chalcogenides, as sources of infrared radiation, have been used for studying chemical reactions. The cost of dysprosium metal has dropped in recent years since the development of ionexchange and solvent extraction techniques, and the discovery of large ore bodies. Thirty-two isotopes and isomers are now known. The metal costs about $6/g (99.9% purity).
Characteristics
Dysprosium, with characteristics similar to most of the other rare-earths, was difficult todiscover. Although dysprosium does not react rapidly with moist air at low temperatures, it does react with water and the halogens at high temperatures. It also reacts in solutions of weakacids. At low temperatures, dysprosium is strongly magnetic.
The Uses of DYSPROSIUM
Dysprosium is most commonly used as in Neodymium-iron-boron high strength permanent magnets. While it has one of the highest magnetic moments of any of the rare earths (10.6uB), this has not resulted in an ability to perform on its own as a practical alternative to Neodymium compositions.
It is however now an essential additive in NdFeB production. It is also used in special ceramic compositions based on BaTiO formulations.
Dysprosium is used, in conjunction with Vanadium and other elements, in making laser materials and commercial lighting. Nanofibers of Dysprosium compounds have high strength and large surface area. Therefore, they can be used to reinforce other materials and as a catalyst.
Recent research has examined the use of Dysprosium in Dysprosium-iron-garnet (DyFeG) and silicon implanted with Dysprosium and Holmium to form donor centers.
Dysprosium Metal is an important additive for NdFeB permanent magnets to raise the Curie temperature and improve temperature coefficiency. Another most promising use of high purity Dysprosium Metal is in the magnetostrictive alloy TEFENOL-D. There are also other applications for some special master alloys. Dysprosium Metal can be further processed to various shapes of ingots, pieces, wires, foils, slabs, rods, discs and powder. It is highly susceptible to magnetization, they are employed in various data-storage applications, such as in hard disks.
The Uses of DYSPROSIUM
There are not many uses for dysprosium. Scientists continue to experiment with it as apossible alloy metal (it has a high melting point) to be mixed with steel to make control rodsthat absorb neutrons in nuclear reactors. There are only a few commercial uses for dysprosium,such as a laser material and as a fluorescence activator for the phosphors used to produce thecolors in the older TV and computer cathode ray tubes (CRTs). When combined with steelor nickel as an alloy, it makes strong magnets.
Definition
A soft malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. One of its few uses is as a neutron absorber in nuclear reactors; it is also a constituent of certain magnetic alloys.
Definition
dysprosium: Symbol Dy. A soft silverymetallic element belonging tothe lanthanoids; a.n. 66; r.a.m.162.50; r.d. 8.551 (20°C); m.p. 1412°C;b.p. 2562°C. It occurs in apatite,gadolinite, and xenotime, fromwhich it is extracted by an ionexchangeprocess. There are sevennatural isotopes and twelve artificialisotopes have been identified. It findslimited use in some alloys as a neutronabsorber, particularly in nucleartechnology. It was discovered by PaulLecoq de Boisbaudran (1838–1912) in1886.
Hazard
Dysprosium nitrate [Dy2(NO3)3] is a strong oxidizing agent and will ignite when in contactwith organic material. Most dysprosium salts are toxic if ingested or inhaled.
Flammability and Explosibility
Flammable
Properties of DYSPROSIUM
| Melting point: | 1412 °C (lit.) |
| Boiling point: | 2567 °C (lit.) |
| Density | 8.559 g/mL at 25 °C (lit.) |
| solubility | soluble in dilute acid solutions |
| form | powder |
| color | Silver-gray |
| Specific Gravity | 8.54 |
| Resistivity | 89 μΩ-cm, 20°C |
| Water Solubility | reacts slowly with H2O; soluble dilute acids [HAW93] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,3515 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7429-91-6(CAS DataBase Reference) |
| EPA Substance Registry System | Dysprosium (7429-91-6) |
Safety information for DYSPROSIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H251:Self-heating substances and mixtures H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P240:Ground/bond container and receiving equipment. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DYSPROSIUM
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

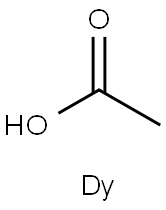
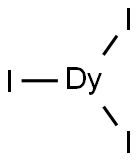
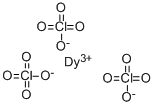
You may like
-
 Dysprosium CAS 7429-91-6View Details
Dysprosium CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium CAS 7429-91-6View Details
Dysprosium CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium CAS 7429-91-6View Details
Dysprosium CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium ingot CAS 7429-91-6View Details
Dysprosium ingot CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium ingot CAS 7429-91-6View Details
Dysprosium ingot CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium pieces CAS 7429-91-6View Details
Dysprosium pieces CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium pieces CAS 7429-91-6View Details
Dysprosium pieces CAS 7429-91-6View Details
7429-91-6 -
 Dysprosium ICP standard CASView Details
Dysprosium ICP standard CASView Details