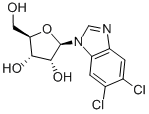
DRB
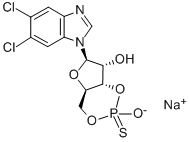
Synonym(s):5,6-Dichloro-1-β-D -ribofuranosylbenzimidazole;5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole - CAS 53-85-0 - Calbiochem;5,6-Dichlorobenzimidazole riboside;5,6-Dichlorobenzimidazole Riboside, DRB, Casein Kinase II Inhibitor VII;DRB
- CAS NO.:53-85-0
- Empirical Formula: C12H12Cl2N2O4
- Molecular Weight: 319.14
- MDL number: MFCD00036785
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 19:43:17
What is DRB?
Description
DRB (53-85-0) is a classic inhibitor of transcription by RNA polymerase II.?A relatively selective inhibitor of Cdk9 (IC50=3 μM), the kinase of the positive transcription elongation factor b (P-TEF-b) required for processive transcription elongation by RNA polymerase II.1,2?Also inhibits casein kinase II, IC50=4-10 μM.?3?Suppresses the SIRT1/CK2α pathway and enhances the radiosensitivity of human cancer cells.4?Kinase-independent activities of Cdk9 such as glucocorticoid receptor modulation are not inhibited by DRB.5
Chemical properties
White Solid
The Uses of DRB
DRB is an inhibitor of RNA synthesis (causes premature termination of transcription).
The Uses of DRB
Inhibits casein kinase II and blocks RNA synthesis. An adenosine analog.5,6-Dichlorobenzimidazole riboside is used as an inhibitor of RNA Polymerase II. It is nucleoside analogue and used in research to study the mechanisms of cellular regulation. Further, it inhibits some protein kinases.
The Uses of DRB
5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside has been used:
- as an inhibitor of RNA polymerase II in mouse melanoma cells
- for the inhibition of cyclin D1 mRNA synthesis in human prostate epithelial cell lines
- in the inhibition of interleukin-2 gene transcription in Jurkat cells
What are the applications of Application
DRB is an adenosine analogue which interrupts DNA transcription
Biochem/physiol Actions
5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB), a nucleoside analog that halts mRNA synthesis by phosphorylation of the C-terminal domain of RNA polymerase II, making it inactive. It also interferes with the DNA topoisomerase II, may modulate response to cytokines and blocks the human immunodeficiency virus (HIV) via RNA modification. It also inhibits cyclin-dependent kinases (CDKs) 7 and 9 and favors apoptosis in leukemic cells. It may serve as a therapeutic agent in treating cancer.
References
1) Baumli?et al.?(2010),?Halogen bonds form the basis for selective P-TEFb inhibition by DRB; Chem. Biol.,?17?931 2) Yamaguchi?et al.?(1998),?Interplay between positive and negative elongation factors: drawing a new view of DRB; Genes Cells,?3?9 3) Zandomeni (1989), Kinetics of inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole; Biochem.J., 262 469 4) Wang?et al.?(2014),?Inhibition of P-TEFb by DRB suppresses SIRT1/CK2α pathway and enhances radiosensitivity of human cancer cells; Anticancer Res.,?34?6981 5) Zhu?et al.?(2014),?A kinase-independent activity of Cdk9 modulates glucocorticoid receptor-mediated gene induction; Biochemistry,?53?1753
Properties of DRB
| Melting point: | 222-224°C |
| Boiling point: | 606.2±65.0 °C(Predicted) |
| Density | 1.5917 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 20 mg/ml) |
| form | White solid |
| pka | 13.23±0.70(Predicted) |
| color | White |
| Water Solubility | Soluble in DMSO to 75mMSoluble in dimethyl sulfoxide, hot ethanol, dimethyl formamide, (1:2) dimethyl formamide:Phosphate buffer saline, water, methanol and pyridine. Slightly soluble in aqueous buffers. |
| λmax | 296nm(lit.) |
| BRN | 39123 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 53-85-0 |
Safety information for DRB
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DRB
| InChIKey | XHSQDZXAVJRBMX-AYLPWXGPSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 5,6-Dichlorobenzimidazole riboside CAS 53-85-0View Details
5,6-Dichlorobenzimidazole riboside CAS 53-85-0View Details
53-85-0 -
 5,6-Dichlorobenzimidazole 1-β-D-Ribofuranoside CAS 53-85-0View Details
5,6-Dichlorobenzimidazole 1-β-D-Ribofuranoside CAS 53-85-0View Details
53-85-0 -
 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside CAS 53-85-0View Details
5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside CAS 53-85-0View Details
53-85-0 -
 5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole CAS 53-85-0View Details
5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole CAS 53-85-0View Details
53-85-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1