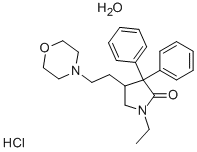
Doxapram hydrochloride monohydrate
Synonym(s):1-Ethyl-4-[2-(4-morpholinyl)ethyl]-3,3-diphenyl-2-pyrrolidinone monohydrochloride monohydrate;Doxapram hydrochloride monohydrate
- CAS NO.:7081-53-0
- Empirical Formula: C24H30N2O2.ClH.H2O
- Molecular Weight: 432.99
- MDL number: MFCD09954698
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:13
What is Doxapram hydrochloride monohydrate?
Description
Doxapram is a central respiratory stimulant. It increases respiration and phrenic nerve activity in anesthetized cats at a dose of 1.0 mg/kg. Doxapram acts through carotid chemoreceptors, lacking activity in anesthetized cats following carotid denervation. Formulations containing doxapram have been used to increase respiration in patients with chronic pulmonary disease, respiratory depression induced by drug overdose, and to stimulate deep breathing post-anesthesia.
Chemical properties
White or almost white, crystalline powder.
Originator
Dopram,Robins,US,1965
The Uses of Doxapram hydrochloride monohydrate
antibacterial
Definition
ChEBI: The monohydrate form of doxapram hydrochloride. A central and respiratory stimulant with a brief duration of action, it is used as a temporary treatment of acute respiratory failure, particularly when superimposed on chronic obstructive pulmonary disease, nd of postoperative respiratory depression. It has also been used for treatment of postoperative shivering.
Manufacturing Process
(A) Preparation of α-(1-ethyl-3-pyrrolidyl)-α,α-diphenylacetonitrile: A
suspension of the sodium salt of diphenylacetonitrile was formed by the
dropwise addition at 50°C of 193 grams (1.0 mol) of diphenylacetonitrile to a
stirred suspension of 43 grams (1.1 mols) of sodium amide in 1 liter of dry
toluene. After addition was complete, the mixture was refluxed for 4 hours
and then, to the refluxing mixture, 1.0 mol of 1-ethyl-3-chloropyrrolidine was
added at a rapid dropwise rate with continuous stirring. After addition was
complete, stirring and refluxing were continued for 3 hours. The mixture was
then cooled and extracted with one normal hydrochloric acid. The aqueous
layer together with an oil layer were separated, made basic with dilute sodium
hydroxide, and extracted with ether. The ethereal solution was dried over
sodium sulfate and concentrated and the residue was distilled in vacuo. The
material crystallized from a 4:1 ethanol-water mixture.
(B) Preparation of 4-(β-chloroethyl)-3,3-diphenyl-1-ethyl-2-pyrrolidinone: A
solution of α,α-diphenyl-α-(1-ethyl-3-pyrrolidyl)-acetonitrile in 70% sulfuric
acid was heated at 130-140°C for 48 hours, poured onto ice, made basic with
sodium hydroxide, and extracted with chloroform. The chloroform solution was
acidified with hydrogen chloride gas, dried over sodium sulfate and
concentrated. The residue was refluxed in 500 ml of thionyl chloride for 3
hours; the resulting solution was concentrated in vacuo; and the residue wascrystallized from isopropyl ether.
(C) Preparation of doxapram hydrochloride [3,3-diphenyl-1-ethyl-4-(2-
morpholino-ethyl)-2-pyrrolidinone hydrochloride monohydrate]: A solution of
25 grams (0.076 mol) of 4-(2-chloroethyl)-3,3-diphenyl-1-ethyl-2-
pyrrolidinone and 13.3 grams (0.153 mol) of morpholine in 500 ml of absolute
ethanol was heated at 95°-120°C for 21 hours in a closed system and
concentrated in vacuo. The residue was dissolved in 300 ml of two normal
hydrochloric acid and extracted with 150 ml of ethyl acetate. A solid
crystallized (13 g) during the extraction and was removed by filtration. MP
217°-219°C. The acid extracts were made basic with sodium hydroxide and
extracted with ether, and the ether solution was concentrated in vacuo and
the residue was suspended in six normal hydrochloric acid. Additional
crystalline product formed and was recrystallized from two normal
hydrochloric acid. Yield, 10 grams; MP 217°-219°C. Total yield, 23 grams
(70%).
brand name
Dopram (Baxter Healthcare).
Therapeutic Function
Respiratory stimulant
General Description
Doxapram, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone hydrochloride hydrate (Dopram), has an obscuremolecular mechanism of action. Overall, it stimulatesrespiration by action on peripheral carotid chemoreceptors.It has use as a respiratory stimulant postanesthetically, afterCNS depressant drug overdose, in chronic obstructive pulmonarydiseases, and in the apneas. Dopram is administeredexclusively by intravenous injection. Because of the benzylalcohol content of the injectable formulation, Dopram mustnever be given to neonates.
Clinical Use
Postoperative respiratory depression
Acute respiratory failure
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: increased risk of arrhythmias with
volatile liquid general anaesthetics - avoid for at least
10 minutes after volatile liquid general anaesthetics.
Metabolism
Doxapram is extensively metabolised in the liver, and the major route of excretion of metabolites and a small amount of unchanged drug is thought to be via bile to the faeces.
Properties of Doxapram hydrochloride monohydrate
| Melting point: | 217-219° |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in water, in alcohol and in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to off-white |
Safety information for Doxapram hydrochloride monohydrate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Doxapram hydrochloride monohydrate
| InChIKey | ZOMBFZRWMLIDPX-UHFFFAOYSA-N |
Doxapram hydrochloride monohydrate manufacturer
Stellar Chemical Laboratories Pvt., Ltd.
AKASH PHARMA EXPORTS
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-Pyrrolidinone, 1-ethyl-4-[2-[(2-hydroxyethyl)amino]ethyl]-3,3-diphenyl-](https://img.chemicalbook.in/CAS/20180629/GIF/1688-76-2.gif)


You may like
-
 7081-53-0 Doxapram hydrochloride 98%View Details
7081-53-0 Doxapram hydrochloride 98%View Details
7081-53-0 -
 7081-53-0 98%View Details
7081-53-0 98%View Details
7081-53-0 -
 Doxapramhydrochloride 99%View Details
Doxapramhydrochloride 99%View Details -
 Doxapram hydrochloride CAS 7081-53-0View Details
Doxapram hydrochloride CAS 7081-53-0View Details
7081-53-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8