DOXACURIUM CHLORIDE
- CAS NO.:106819-53-8
- Empirical Formula: C56H78Cl2N2O16
- Molecular Weight: 1106.13
- MDL number: MFCD00906167
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:29
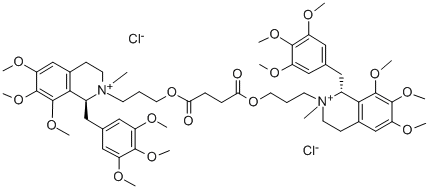
What is DOXACURIUM CHLORIDE?
Description
Doxacurium chloride is an injectable, noncumulative, nondepolarizing neuromuscular blocking agent which exhibits no significant cardiovascular effects. It is a mixture of (lR, l′S, 2S, 2′R), (lR, l′R, 2S, 2′s) and (lS, l′S, 2R, 2′R) isomers of a bis-benzylisoquinolinium diester.Doxacurium chloride provides satisfactory intubation with duration of action similar to d-tubocurarine or pancuronium. However, it is reported to be 2 to 3 times as potent as pancuronium.
Originator
Wellcome (United Kingdom)
The Uses of DOXACURIUM CHLORIDE
Doxacurium Chloride is a nondepolarizing neuromuscular blocking agent that is minimally hydrolyzed by human plasma cholinesterase.
The Uses of DOXACURIUM CHLORIDE
Neuromuscular blocking agent.
brand name
Nuromax (Abbott).
General Description
The molecular structure of doxacuriumchloride, 1,2,3,4-tetrahydro-2-(3-hydroxypropyl)-6,7,8-trimethoxy2-methyl-1-(3,4,5-trimethoxybenzyl) isoquinoliniumchloride succinate (Nuromax), provides thepossibility for 10 stereoisomers: 4 d,l pairs and two mesoforms. Of the 10 stereoisomers, 3 are all-trans configuration,and these are the only active ones. Doxacurium chlorideis a long-acting nondepolarizing blocking agent. The drugdiffers from drugs such as gallium and pancuronium in thatit has no vagolytic activity. It is used as a skeletal musclerelaxant in surgical procedures expected to last longer than90 minutes.
Pharmacokinetics
Doxacurium is a mixture of three trans, trans-stereoisomers, a dl pair [(1R,1′R,2S,2′S) and (1S,1′S,2R,2′R)] and a meso form (1R,1′S,2S,2′R). Doxacurium is hydrophilic, has a small volume of distribution, and is distributed primarily to extracellular fluids. It is not metabolized by plasma cholinesterase or hepatic enzymes and does not undergo Hofmann elimination.
Safety information for DOXACURIUM CHLORIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![PHENOL, 2-METHOXY-5-[2-(3,4,5-TRIMETHOXYPHENYL)ETHYL]-](https://img.chemicalbook.in/CAS/GIF/95041-90-0.gif)
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
