Dotinurad
- CAS NO.:1285572-51-1
- Empirical Formula: C14H9Cl2NO4S
- Molecular Weight: 358.2
- Update Date: 2024-11-19 15:53:33
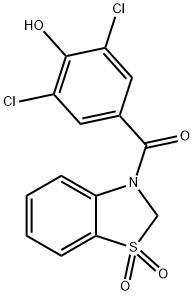
What is Dotinurad?
Description
Dotinurad is an organic compound with the chemical Name (3,5-dichloro-4-hydroxyphenyl)(1,1-dioxidobenzo[d]thiazol-3(2H)-yl)methanone. This compound is a newer urate-lowering agent that suppresses uric acid reabsorption through the selective inhibition of urate transporter 1 (URAT1) in the proximal renal tubules, and it was first approved in Japan in 2020 for the treatment of hyperuricemia, irrespective of gout[1].
The Uses of Dotinurad
Dotinurad is a useful drug for treating hyperuricemia and gout.
Pharmacokinetics
The plasma concentration of dotinurad increased in a dose-dependent manner with Cmax and AUC0–24h values of 107 μg/ml and 780 μg.h/ml, respectively, at a dose of 30 mg/kg. Dotinurad dose-dependently lowered the plasma urate levels with its maximum effect at 8 h. Changes in plasma urate level between 0 and 8 h (ΔPUA) were lower than that of control by 0.28, 0.97 (p < 0.05), and 1.79 mg/dl (p < 0.01), at doses of 1, 5, and 30 mg/kg, respectively. Furthermore, dotinurad dose-dependently increased FEUA. The 0–4 h FEUA increased by 180%, at a dose of 30 mg/kg compared to the control (p < 0.01)[3].
Advantages
Dotinurad, as a selective urate reabsorption inhibitor, is anticipated to be more effective in inhibiting urate reabsorption compared to conventional urate-lowering agents. It demonstrates noninferiority to benzbromarone or febuxostat in reducing serum urate levels in hyperuricemic patients with or without gout. Moreover, its effectiveness remains uncompromised in patients with mild to moderate renal or hepatic impairment. In a long-term study, a majority of patients achieved the target serum urate level of ≤6 mg/dL when administered a maintenance dose of 2 or 4 mg once daily. No safety concerns, such as liver injury, were observed. Consequently, Dotinurad is expected to serve as a new therapeutic option for hyperuricemic patients, regardless of the presence of gout [2].
References
[1] Tanaka A, et al. Clinical effects of a selective urate reabsorption inhibitor dotinurad in patients with hyperuricemia and treated hypertension: a multicenter, prospective, exploratory study (DIANA). European Journal of Medical Research, 2023; 238.
[2] Ishikawa T, et al. Dotinurad: a novel selective urate reabsorption inhibitor for the treatment of hyperuricemia and gout. Expert Opinion on Pharmacotherapy, 2021; 22: 1397-1406.
[3] Taniguchi T, et al. Pharmacological Evaluation of Dotinurad, a Selective Urate Reabsorption Inhibitor. Journal of Pharmacology and Experimental Therapeutics, 2019; 162-170.
Properties of Dotinurad
| Melting point: | 188 - 191°C |
| Boiling point: | 614.8±55.0 °C(Predicted) |
| Density | 1.662±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly, Sonicated), DMSO (Sparingly) |
| form | Solid |
| pka | 4.60±0.25(Predicted) |
| color | White to Off-White |
Safety information for Dotinurad
Computed Descriptors for Dotinurad
Abamectin manufacturer
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Dotinurad 97%View Details
Dotinurad 97%View Details -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4