DL-Mandelic acid
- CAS NO.:90-64-2
- Empirical Formula: C8H8O3
- Molecular Weight: 152.15
- MDL number: MFCD00064250
- EINECS: 202-007-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
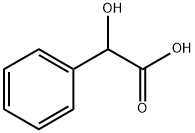
What is DL-Mandelic acid ?
Chemical properties
White rhomboidal crystals, decompose when exposed to light. Soluble in ether and isopropanol, soluble in ethanol and water; R-form melting point is 133°C, [α]-159.73° (ethanol), gradually racemic at 160°C. S-form melting point is 133.8°C, [α]+156.57° (water).
The Uses of DL-Mandelic acid
DL-Mandelic acid may be used as an analytical reference standard for the determination of DL-mandelic acid in:
Human urine samples by high performance liquid chromatography (HPLC) equipped with ultraviolet (UV) detector.
Rat urine samples by gas chromatography-mass spectrometry (GC-MS) with selected ion monitoring (SIM) detection.
The Uses of DL-Mandelic acid
Organic synthesis, medicine (urinary antiseptic).
Background
Mandelic acid is an approved aromatic, alpha hydroxy acid . Mandelic acid is used as an ingredient in cosmetics and drug products applied topically.
Definition
ChEBI: Mandelic acid is a 2-hydroxy monocarboxylic acid that is acetic acid in which two of the methyl hydrogens are substituted by phenyl and hydroxyl groups. It has a role as an antibacterial agent and a human xenobiotic metabolite. It is a 2-hydroxy monocarboxylic acid and a member of benzenes. It derives from an acetic acid. It is a conjugate acid of a mandelate. Exists in stereoisomeric forms. The properties are those of the dl-form.
Preparation
Mandelic acid can be prepared by the hydrolysis of amygdalin with sulfuric acid or of mandelonitrile with hydrochloric acid. The mandelonitrile can be prepared by the action of hydrocyanic acid on benzaldehyde, and by the action of sodium or potassium cyanide on the sodium bisulfite addition product of benzaldehyde. The procedure described differs from earlier methods in that the sodium bisulfite addition compound of benzaldehyde is prepared in the presence of sodium cyanide and the nitrile is formed immediately.
synthesis of mandelic acid
General Description
DL-Mandelic acid is an aromatic hydrocarbon metabolite of styrene.
Hazard
Toxic by ingestion.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intramuscular route. Moderately toxic by ingestion. Continued absorption can cause kidney irritation. When heated to decomposition it emits acrid smoke and irritating fumes.
Preparation
DL-Mandelic acid stands as an aromatic alpha hydroxy acid (AHA) resulting from the hydrolysis of mandelonitrile.
Metabolism
Not Available
Properties of DL-Mandelic acid
| Melting point: | 119-121 °C (lit.) |
| Boiling point: | 214.6°C (rough estimate) |
| alpha | [α]D20 -0.5~+0.5° (c=2, H2O) |
| Density | 1.30 |
| vapor pressure | 0.01 Pa (50 °C) |
| refractive index | 1.4810 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 139g/l |
| form | Crystals or Crystalline Powder |
| pka | 3.85(at 25℃) |
| color | White |
| PH | 2.3 (10g/l, H2O) |
| Odor | faint odor |
| optical activity | [α]/D 0±1°, c = 5 in H2O |
| Water Solubility | 150 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,5717 |
| BRN | 510011 |
| CAS DataBase Reference | 90-64-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetic acid, «alpha»-hydroxy-(90-64-2) |
| EPA Substance Registry System | Mandelic acid (90-64-2) |
Safety information for DL-Mandelic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DL-Mandelic acid
| InChIKey | IWYDHOAUDWTVEP-UHFFFAOYSA-N |
DL-Mandelic acid manufacturer
SYNGREEN LIFESCIENCES PVT.LTD
JSK Chemicals
SS Reagents and Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 90-64-2 DL-Mandelic acid 98%View Details
90-64-2 DL-Mandelic acid 98%View Details
90-64-2 -
 Pregabalin Impurity C 90-64-2 98%View Details
Pregabalin Impurity C 90-64-2 98%View Details
90-64-2 -
 DL-Mandelic Acid CAS 90-64-2View Details
DL-Mandelic Acid CAS 90-64-2View Details
90-64-2 -
 DL-Mandelic acid CAS 90-64-2View Details
DL-Mandelic acid CAS 90-64-2View Details
90-64-2 -
 DL-Mandelic Acid CAS 90-64-2View Details
DL-Mandelic Acid CAS 90-64-2View Details
90-64-2 -
 DL-MANDELIC ACID Extra Pure CAS 90-64-2View Details
DL-MANDELIC ACID Extra Pure CAS 90-64-2View Details
90-64-2 -
 DL-Mandelic acid 98% CASView Details
DL-Mandelic acid 98% CASView Details -
 DL-MANDELIC ACID CAS 90-64-2View Details
DL-MANDELIC ACID CAS 90-64-2View Details
90-64-2